Schneider Research Group
Our Team
Our Research
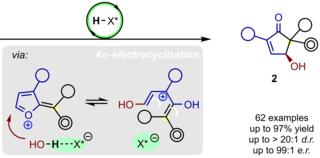
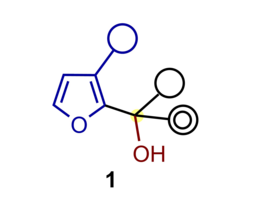
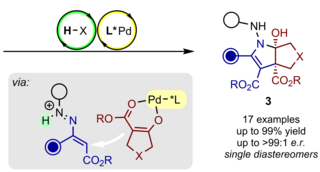
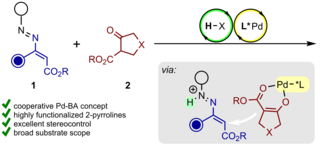
We study, develop, and apply novel and useful, catalytic, and highly stereoselective bond-forming processes. They are intended to provide straightforward access to complex and highly valuable chemical structures represented in biologically active natural products and medicinally relevant molecules.
Our Publications
Positions
If you are interested in working in our group, you can send your application (CV, recommendation, list of publication and presentation) to Prof. C. Schneider by E-mail or postal mail.
Teaching
Here you can find further information about lectures, seminars, and internships and about registration for Bachelor, Master, and Ph.D thesis.
„Highlights in Natural Products Synthesis" (13-122-0321)
Summer Semester 2022 for Master Chemie (Choice: 13-122-0321):
Information will become available as soon as the Summer Semester 2022 starts. For questions, please contact Dr. Marcel Sickert.
"Organisch-Chemische Reaktionsmechanismen"- "OC-II" (13-111-0341-N)
The course is given in German.
Winter Semester 2023/2024 - for 3rd Semester of Bachelor Chemie: Join the Moodle-course with the key you got by registering for the Module via Almaweb. For further questions, please contact Dr. Marcel Sickert.
„Organisch-chemische Reaktionsmechanismen” OC-II (13-BCH-0310)
The course is given in German.
Winter Semester 2023/2024 - for 3rd Semester of Bachelor Biochemie: Join the Moodle-course with the key you got by registering for the Module via Almaweb. For further questions, please contact Dr. Marcel Sickert.
„Stereoselektive Synthesemethoden" (13-121-0317)
Winter Semester 2023/2024 for Master of Chemistry (Choice: 13-121-0317):
Information will become available as soon as the Winter Semester 2023/2024 starts. For questions, please contact Dr. Marcel Sickert.
„OC-Grundpraktikum" (13-111-0341-N.P)
The course is given in German. Winter Semester 2021/2022 for 3th Semester of Bachelor Chemie:
Join the Moodle-course with the key you got after passing the OCI-exam. For further questions, please contact Dr. Marcel Sickert.