Welcome to our website of the Schneider research group. Here you can find further information about our research.
Our Research Activity
From Stereoselective Catalysis to Natural Product Synthesis
The central theme of our research activities is synthetic organic chemistry. We study, develop, and apply novel and useful, catalytic, and highly stereoselective processes for important carbon-carbon and carbon-heteroatom bond-forming reactions. As chiral catalysts we use small organic molecules, such as chiral Brønsted acids and amines, as well as chiral transition metal complexes which control the correct absolute configuration of the products. These processes are subsequently utilized for the straightforward assembly of complex molecular architectures with a defined three-dimensional structure. As ideal testing ground in this regard serve biologically active natural products und medicinally relevant molecules.
Silyl dienolates are nucleophilic C4-units. Under Lewis or Brønsted acid catalysis they can be added onto aldehydes, imines and Michael acceptors and expand the molecule backbone by these four carbon atoms. The reaction products itself contain up to two new stereogenic centres, are highly functionalized, and prone for further transformations.
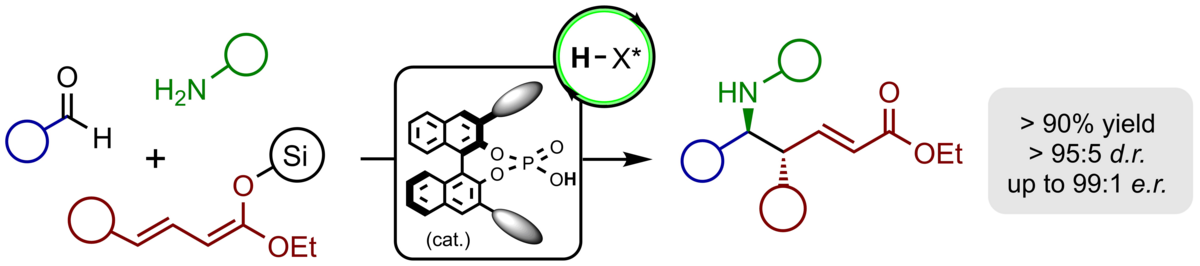
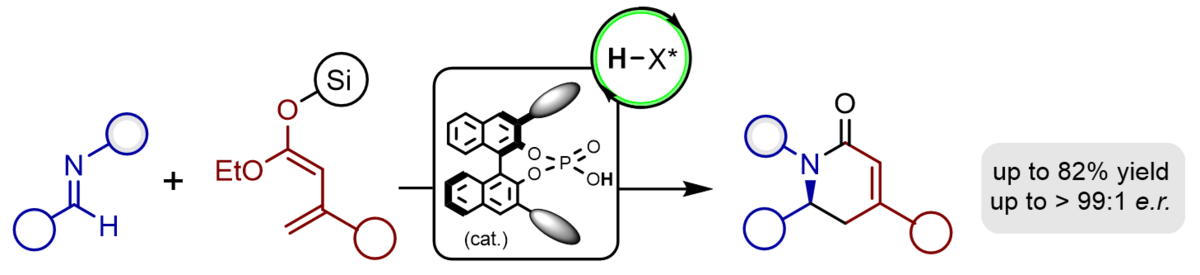
We have recently established the first catalytic, highly stereoselective vinylogous Mannich reactions of acyclic vinylketene silyl-O,O-acetals with imines which furnish valuable unsaturated amino esters with excellent yields, complete regioselectivity and excellent enantio- and diastereoselectivity. As chiral catalyst we employ a BINOL-based phosphoric acid which upon protonation of the imine produces a chiral, highly reactive contact ion pair.
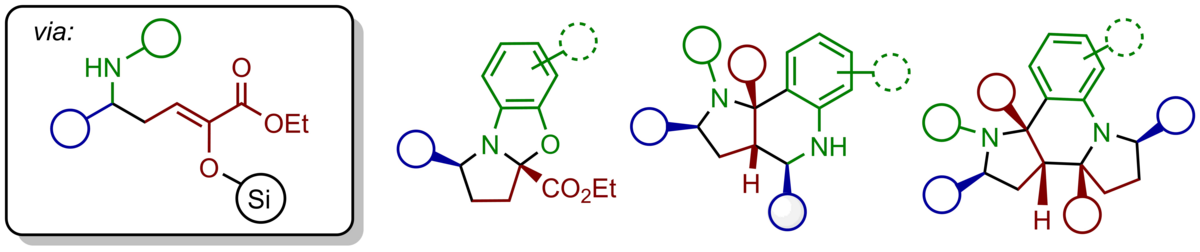
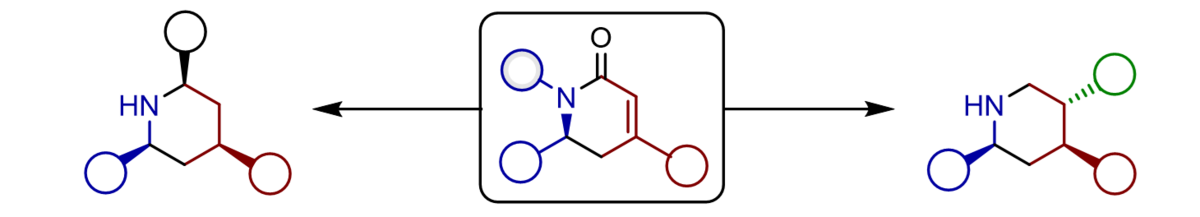
The application of α-silyloxy-substituted silyl dienolates provides vinylogous Mannich products bearing reactive silyl enol ether groups prone for additional modifications. Both the nucleophilic β-position and the electrophilic a-carbonyl group upon hydrolysis can be used to access a variety of multicyclic heterocycles just by modification of the reaction conditions.
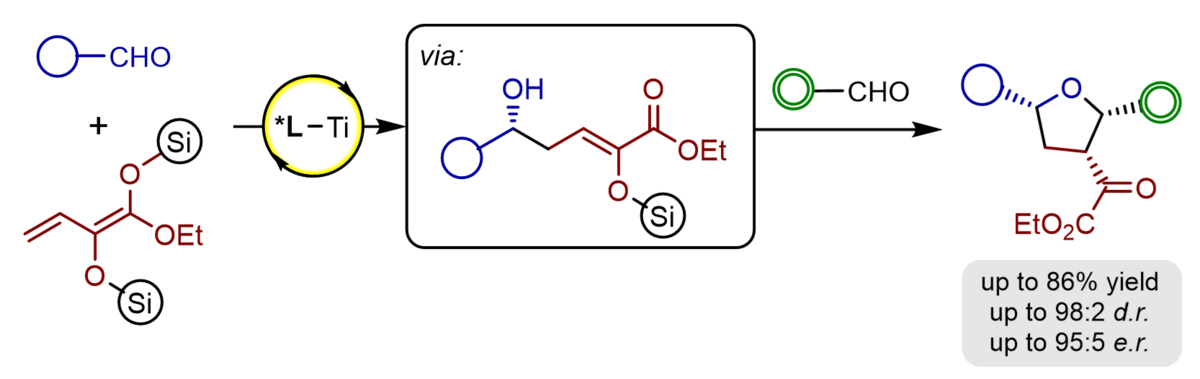
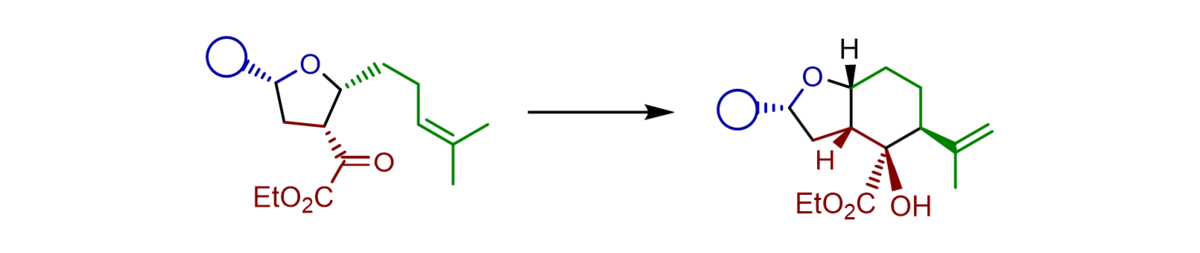
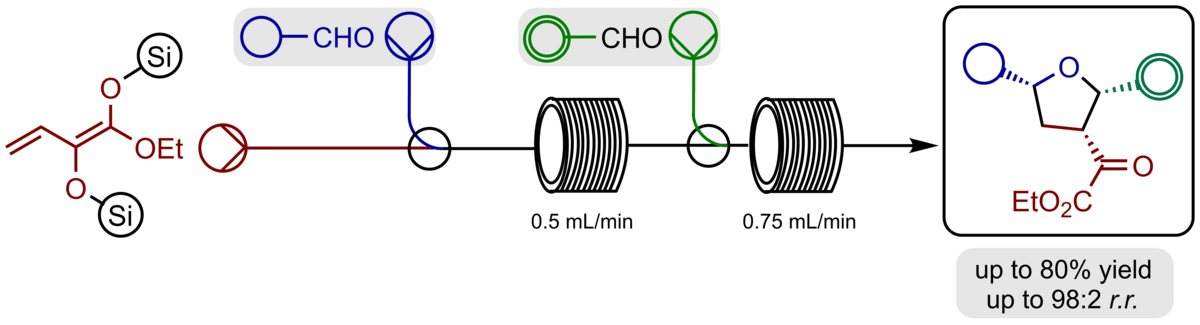
An efficient access to functionalized and enantiomerically highly enriched tetrahydrofurans has been developed. The sequential procedure included an an asymmetric Titanium(IV)-BINOLate catalysed aldol reaction of aldehydes with α-silyloxy-substituted silyl dienolates followed by Prins-cyclisation generated products bearing three stereogenic centres in typical good yields, high γ-regioselectivities, very good diastereoselectivities, and excellent enantioselectivities up to 95:5 e.r. With further functionalized aldehydes the sequence could be expanded by a carbonyl-ene reaction to furnish octahydrobenzofuranes bearing two additional stereogenic centres.
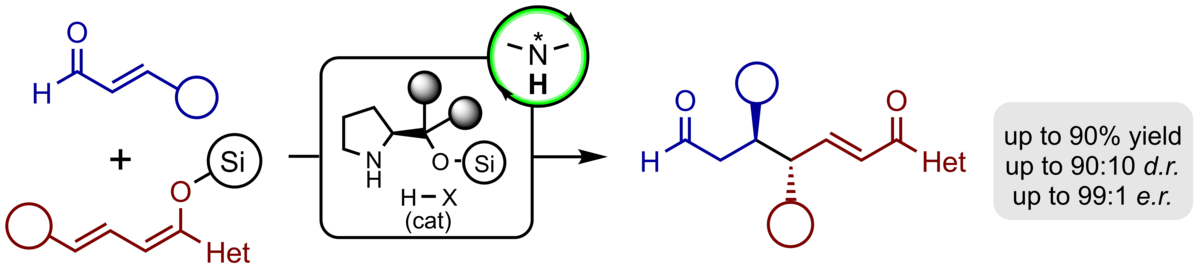
We have established the first catalytic, enantioselective, vinylogous Michael reaction of silyl dienol ethers and enals which proceeds with exceptional regio- as well as enantioselectivity. In the presence of the Jørgensen-Hayashi catalyst the desired products are obtained in typically good yields, complete γ-regioselectivity, and excellent enantioselectivity exceeding 97% ee in all cases studied. Moreover, with γ-substituted nucleophiles a second stereogenic centre can be easily installed with good diastereoselectivity of >10:1.
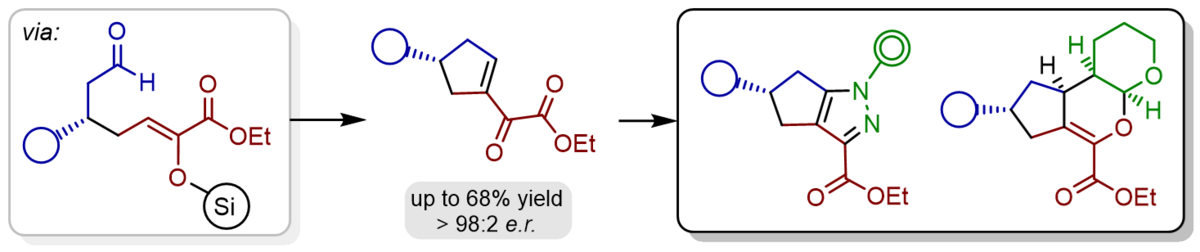
In the case of using α-silyloxy-substituted silyl dienolates for the catalytic, enantioselective, and vinylogous Michael reaction an additional intramolecular aldol condensation was observed furnishing highly valuable chiral cyclopentenes in good yields and with high enantioselectivities. Furthermore, the α-keto ester moiety of the product can be easily converted with a variety of reagents to obtain highly functionalized chiral cyclopentanes and multicyclic heterocycles.
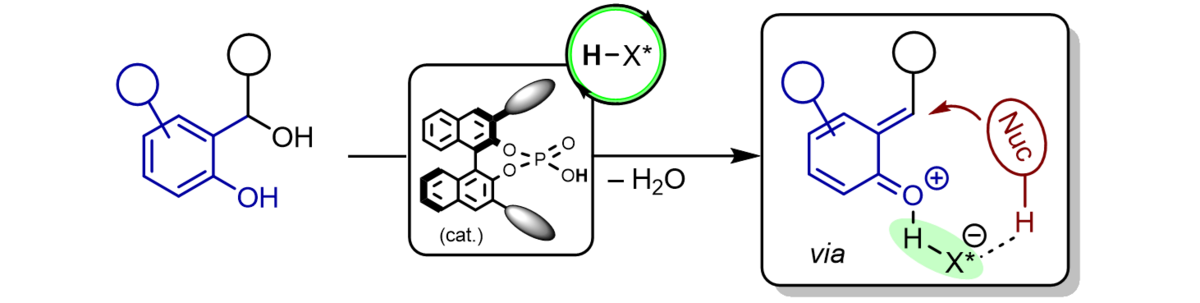
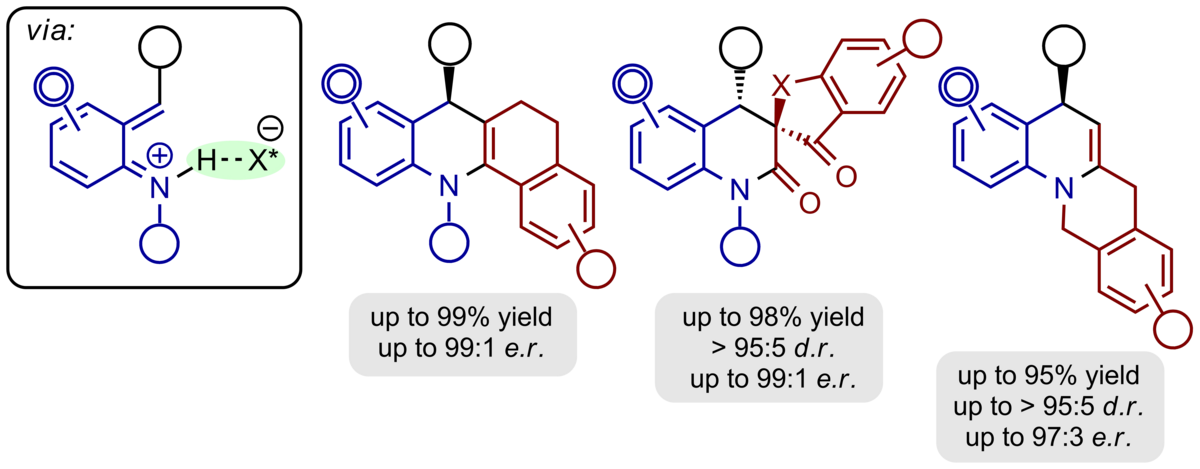
Ortho-quinone methides are highly reactive synthetic intermediates which have only recently been used to a greater extent in organic chemistry. They are easily available from a variety of precursors and react as polarized, electron-poor 1-oxabutadienes mostly with electron-rich π-components in hetero-Diels-Alder reactions with inverse electron demand or with nucleophiles in conjugate addition reactions both under re-aromatization.
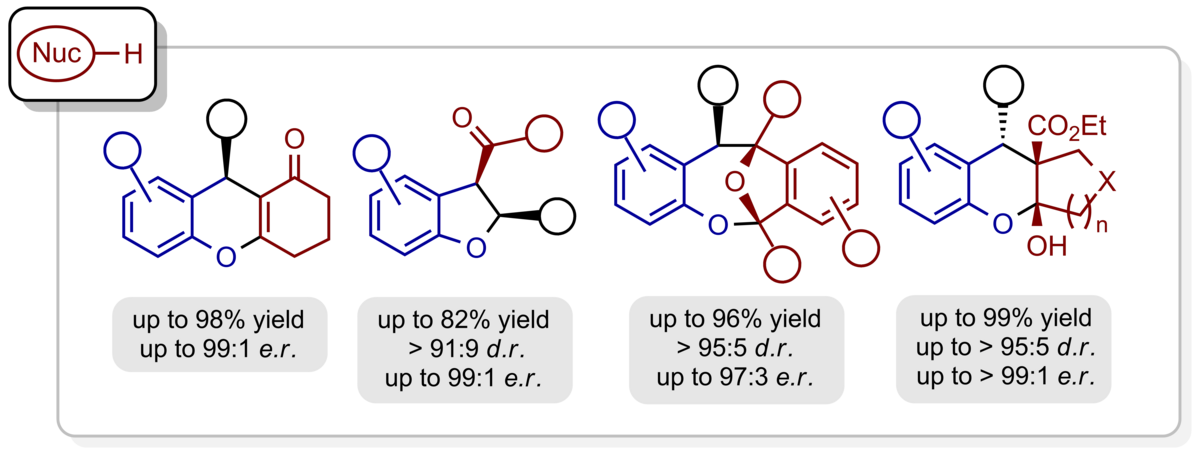
Using chiral phosphoric acids as powerful organocatalysts we have been able to generate hydrogen-bonded ortho-quinone methides in situ to which a variety of electronrich π-nucleophiles can be added in a cycloaddition or conjugate addition event with excellent enantioselectivity. β-Dicarbonyl compounds, enamides, enolrich aldehydes, indoles, phenols, and diazo esters are among the most suitable reaction partners to furnish benzannulated 5-, 6-, and 7-membered oxygen heterocycles as enantiomerically highly enriched compounds.
Ortho-quinone methide imines are characterized by their close relationship to ortho-quinone methides. They are accessible from the respective amino alcohols by chiral phosphoric acid-catalysis and are converted into hexahydroacridines, dihydroquinolones, and benzannulated quinolizidines with excellent enantioselectivity.
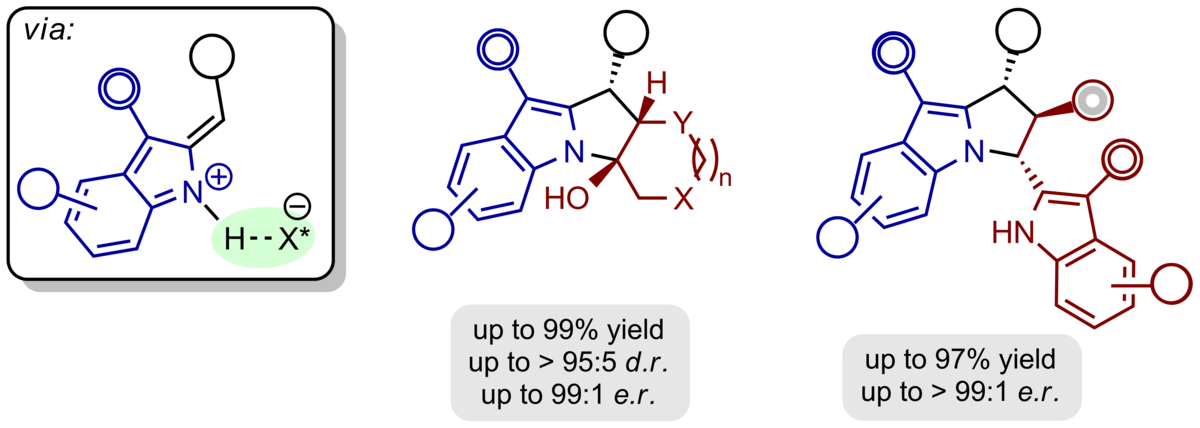
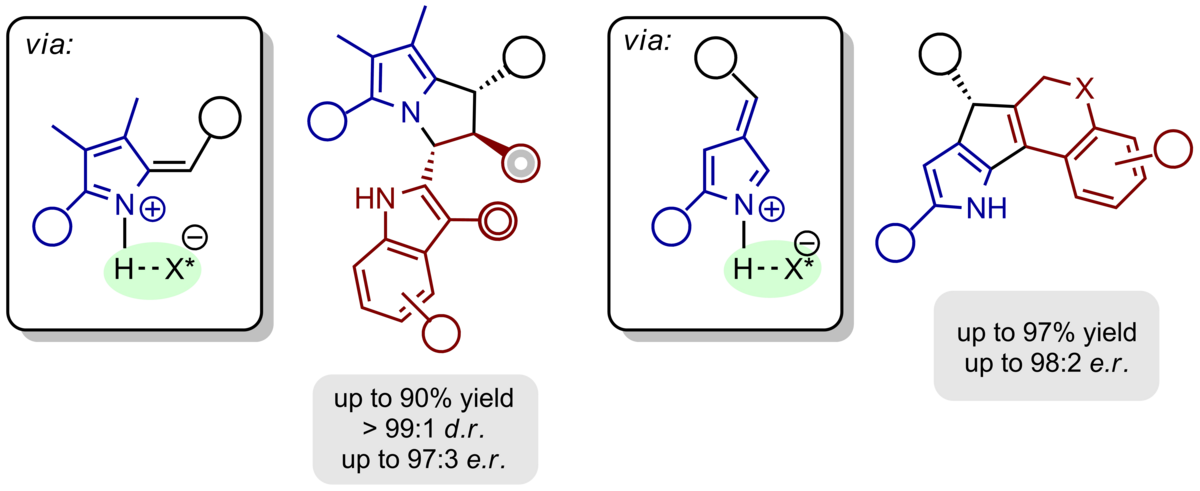
The aforementioned concept of the ortho-quinone methide chemistry can be easily extended to heterocyclic motifs. Thus, hydrogen-bonded, chiral alkylidene 2H-indoles and 2H-pyrroles are produced upon acid-catalysed dehydration from the respective alcohols. They react easily with electronrich π-nucleophiles to produce pyrrolo[1,2-a]indoles, 1H‑pyrrolizines, and cyclopenta[b]pyrroles with defined absolute and relative configuration.
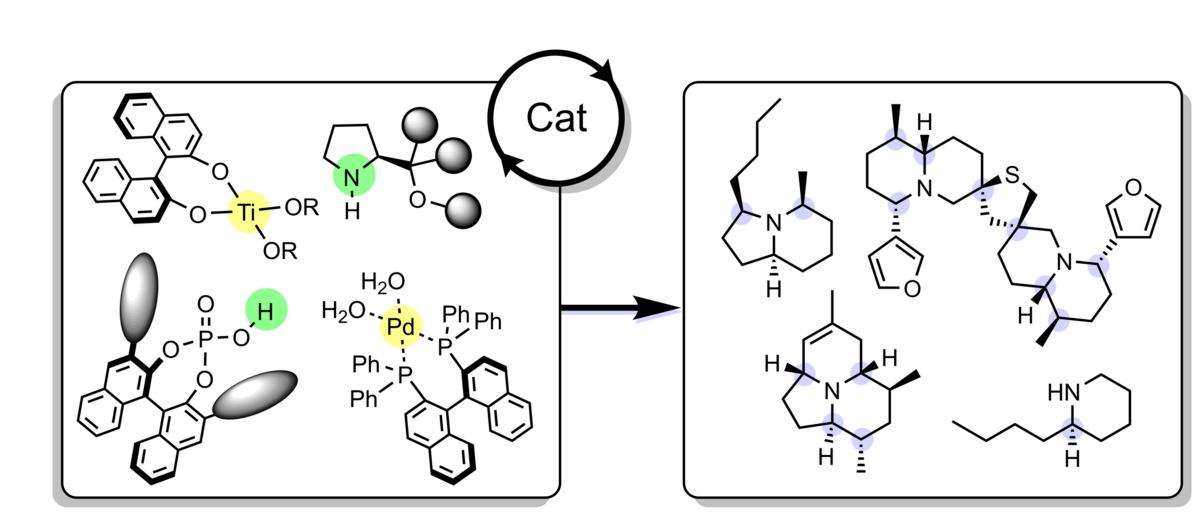
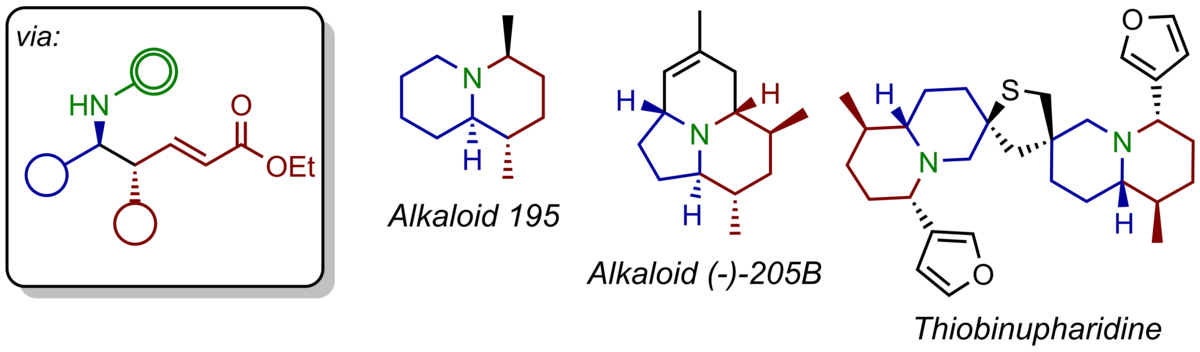
Taking advantage of our enantioselective vinylogous Mannich reaction a range of alkaloids have been prepared in enantiomerically highly enriched form. In particular, bicyclic nitrogen heterocycles such as indolizidines and quinolizidines commonly found as constituents of frog skins have been obtained as single-enantiomer compounds via a straightforward, modular-type strategy. Just recently, the same strategy was applied to the currently shortest enantioselective synthesis of the tricyclic alkaloid (-)-205B. Currently under investigation is a total synthesis of the dimeric nuphar alkaloid thiobinupharidine.
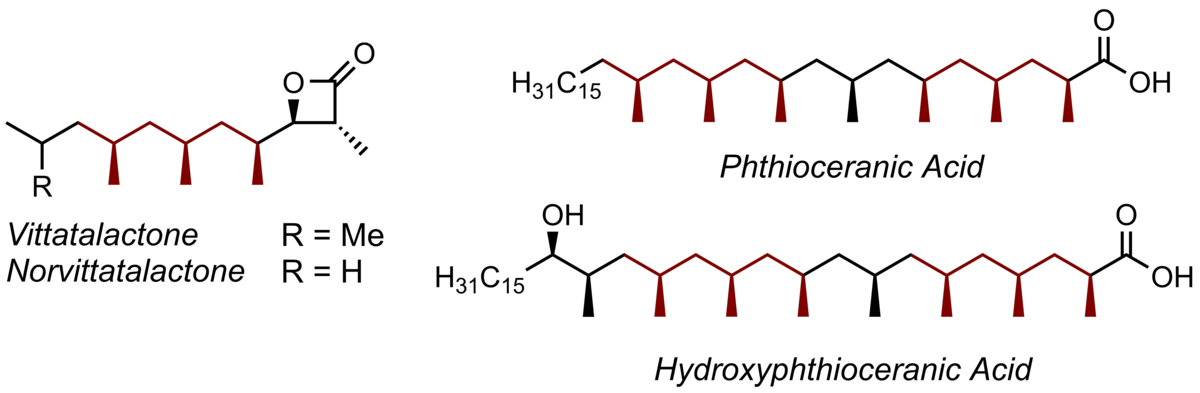
Another class of natural products that we have synthesized recently are polydeoxypropionates. They are produced by bacteria, funghi, and plants and display potent and diverse biological activities. They are characterized by an alkyl chain substituted with methyl groups at every second carbon atom differing from the related polypropionates in the lack of hydroxyl groups at the other carbon atoms. In the light of their significant biological activity a good amount of work has been directed at their stereoselective assembly worldwide.
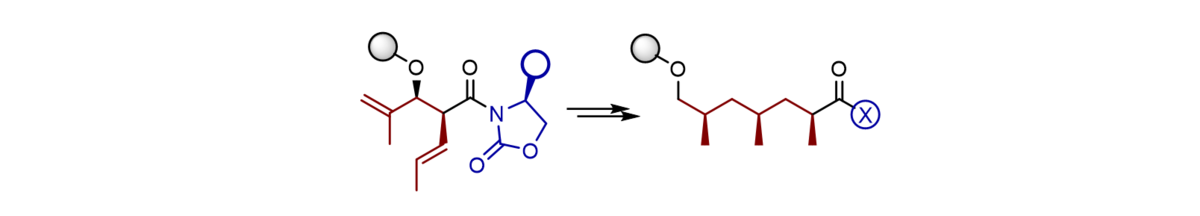
We have developed a novel and conceptually different synthetic access to this product class which for the first time does not rely on an iterative synthesis, but rather provides trideoxypropionate subunits directly which can be coupled in a highly convergent manner to furnish long-chain polydeoxy-propionates. The insect pheromones vittatalactone and norvittatalactone and the lipid components phthioceranic acid and hydroxyphthioceranic acid were assembled as single stereoisomers following this concept.