Below you can find a detailed list of our publications as well as a gallery containing our published cover pictures in peer-reviewed journals.
Cover Pictures
Publication List
62) S. Schmidt; H. Westphal; S. Lama; M. Polack; C. Weise; T. Oestereich; R. Warias; T. Gulder; D. Belder; Development of an Automated Platform for Monitoring Microfluidic Reactors through Multi-Reactor Integration and Online (Chip-)LC/MS-Detection; React. Chem. Eng. 2024; 10.1039/d4re00004h. Fulltext
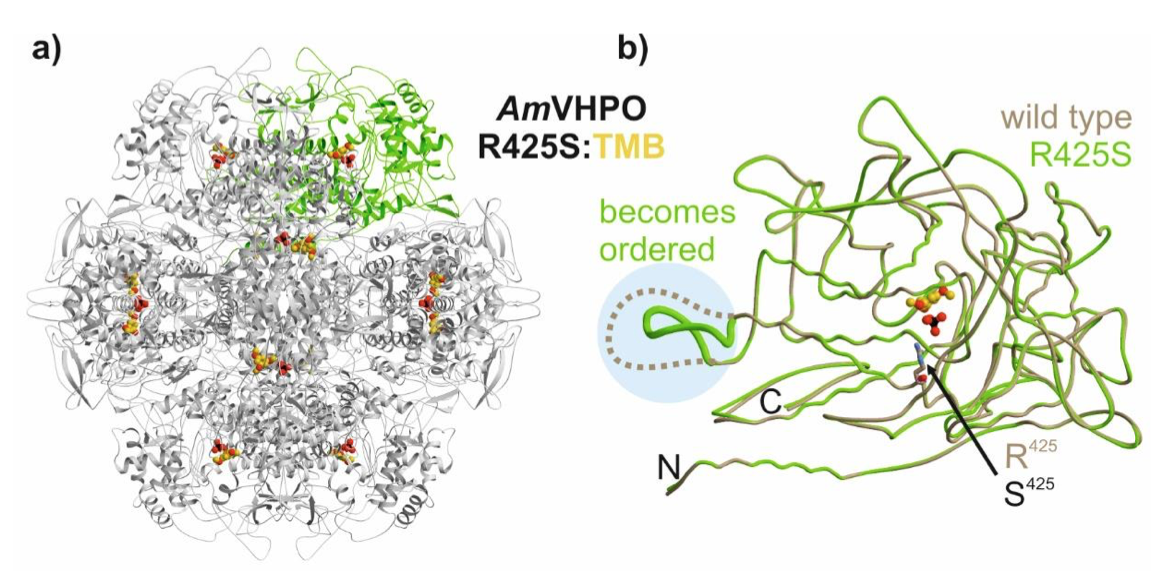
61) P. Zeides; K. Bellmann-Sickert; R. Zhang; C. Seel; V. Most; C. Schoeder; M. Groll; T. Gulder; Unraveling the Molecular Basis of Substrate Specificity and Halogen Activation in Vanadium-Dependent Haloperoxidases; ChemRxiv 2023; 10.26434/chemrxiv-2023-7l30h. Fulltext
60) A. Arnold; J. Binder; M. Kretzschmar; T. Gulder; Alkene versus Aryl Chlorination in Asymmetric Hypervalent Iodine Catalysis: A Case Study; Synlett 2023; 10.1055/a-2201-7326. Fulltext
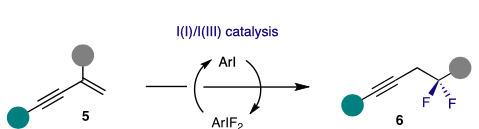
59) R. C. Epplin; T. Gulder; Enyne difluorination; Nat. Chem. 2023; 10.1038/s41557-023-01352-5. Fulltext
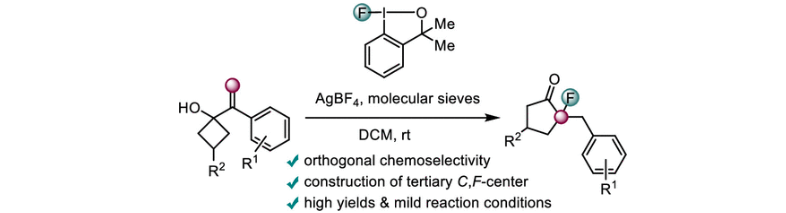
58) P. Zhao; W. Wang; T. Gulder; Hypervalent Fluoro-iodane-Triggered Semipinacol Rearrangements: Synthesis of α-Fluoro Ketones; Org. Lett. 2023; 25; 6560-6565. Fulltext
57) J. Matysik; L. Gerhards; T. Theiss; L. Timmermanns; P. Kurle-Tucholski; G. Musabirova; R. Qin; F. Ortmann; I.A. Solov'yov; T. Gulder; Spin Dynamics of Flavoproteins; Int. J. Mol. Sci. 2023, 24, 8218. Fulltext
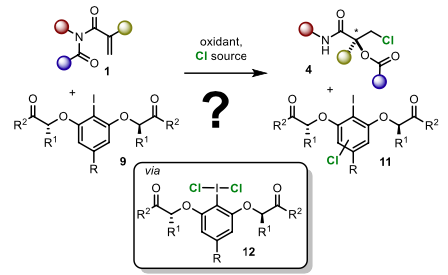
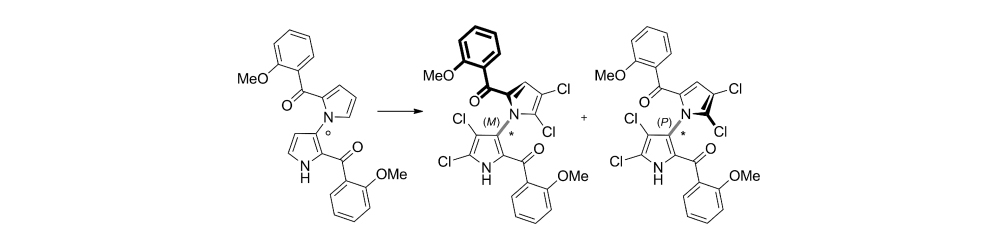
56) J. Binder; A. Biswas; T. Gulder; Biomimetic Chlorine-Induced Polyene Cyclizations harnessing Hypervalent Chloroiodane-HFIP Assemblies; Chem. Sci. 2023, 14, 3907-3912. Fulltext
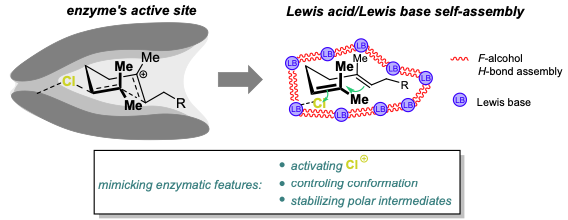
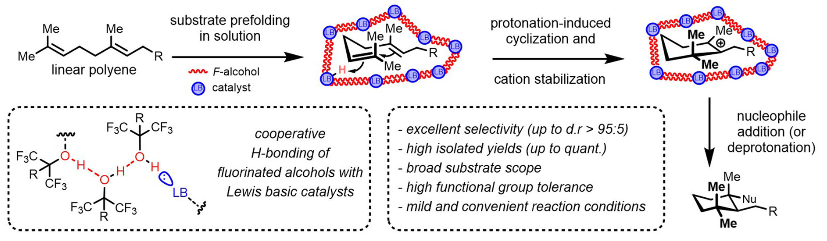
55) A.M. Arnold; P. Dullinger; A. Biswas; C. Jandl; D. Horinek; T. Gulder; Enzyme-like Polyene Cyclizations Catalyzed by Dynamic, Self-Assembled, Supramolecular Fluoro Alcohol-Amine Clusters; Nat. Commun. 2023, 14, 813. FULLTEXT
54) M. Kretzschmar; T. Gulder; Discovering the Site-Selective Umpolung of Ketones triggered by Hypervalent Fluoro Iodanes – Why Investigating Side Reactions Matters!; Synlett 2022, 34, 405 - 413. FULLTEXT
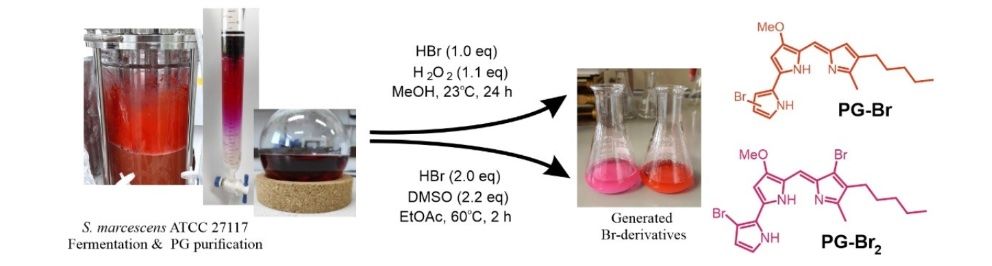
53) J. Lazic; S. S. Bogojevic; S. Vojnovic; I. Aleksic; D. Milivojevic ; M. Kretzschmar; T. Gulder; M. Petkovic; J. Nikodinovic-Runic; Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117; Molecules 2022, 27, 3729. FULLTEXT
52) A. Das; C. Weise; M. Polack; R. D. Urban; B. Krafft; S. Hasan; H. Westphal; R. Warias; S. Schmidt; T. Gulder; D. Belder; On-the-Fly Mass Spectrometry in Digital Microfluidics Enabled by a Microspray Hole: Toward Multidimensional Reaction Monitoring in Automated Synthesis Platforms; J. Am. Chem. Soc. 2022, 144, 10353–10360. FULLTEXT
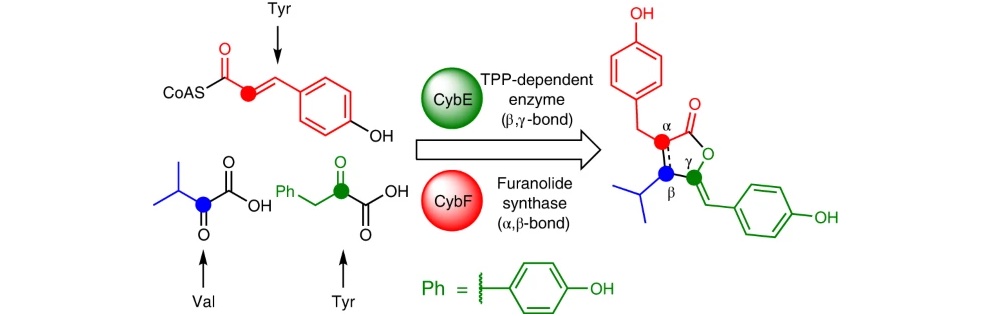
51) P. D'Agostino, C. J. Seel, X. Ji, T. Gulder, T. A. M. Gulder; Biosynthesis of cyanobacterin, a paradigm for furanolide core structure assembly; Nat Chem Biol 2022, 18, 652 – 658. FULLTEXT
50) D. Stierli, M. Eberle, C. Lamberth, O. Jacob, D. Balmer, T. Gulder; Quarternary a-cyanobenzylsulfonamides: a new subclass of CAA fungicides with excellent anti-Oomycetes activity; Bioorg. Med. Chem. 2021, 30, 115965. Fulltext
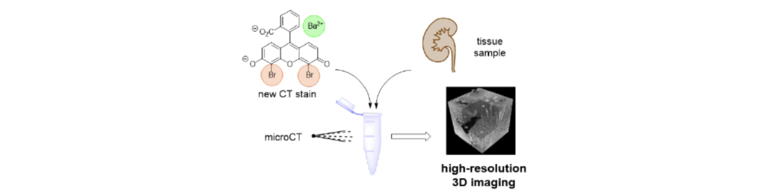
49) M. Busse, J. P. Marciniszyn, S. Ferstl, M. A. Kimm, F. Pfeiffer, T. Gulder; 3D‐Non‐Destructive Imaging through Heavy Metal‐Eosin Salt Contrast Agents; Chem. Eur. J. 2021, 27, 4561 - 4566. Fulltext
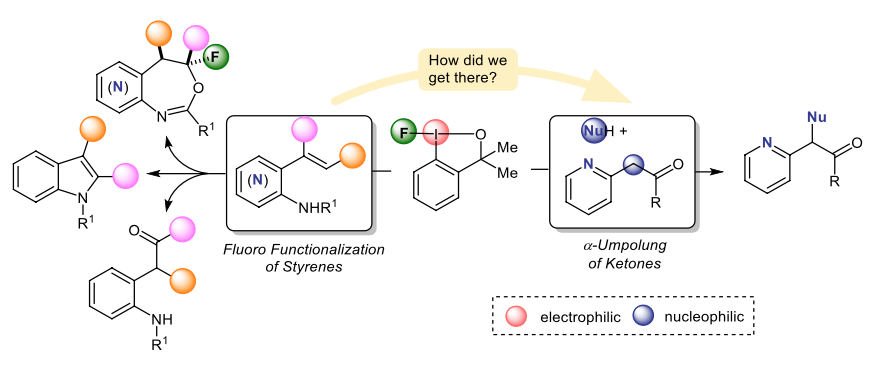
48) G. M. Kiefl; T. Gulder; α-Functionalization of Ketones via a Nitrogen Directed Oxidative Umpolung; J. Am. Chem. Soc. 2020; 142, 20577–20582. Fulltext
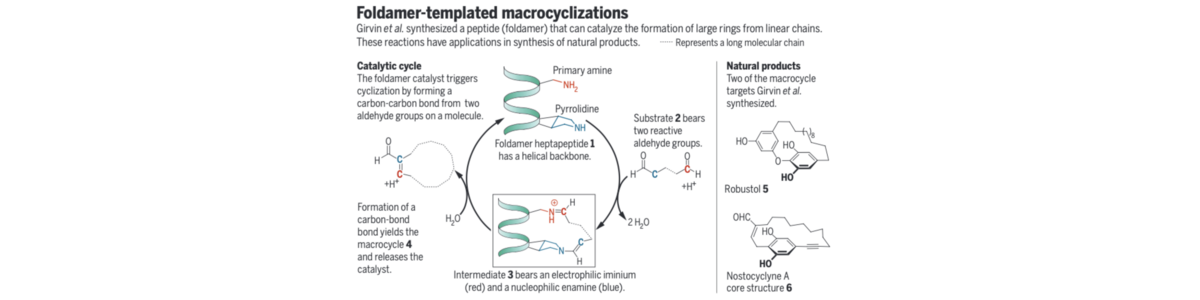
47) A. G. Collar, T. Gulder; Peptidic catalysts for macrocycle synthesis; Science 2019, 6472, 1454. Fulltext
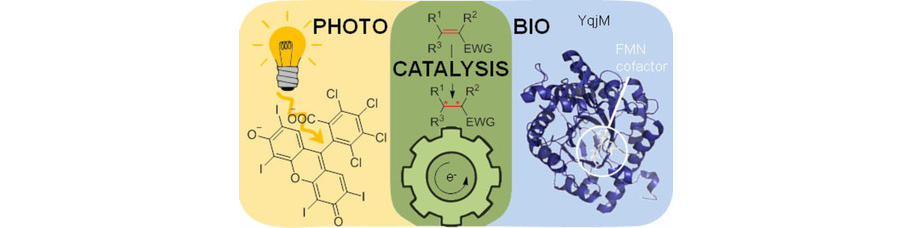
46) C. J. Seel, T. Gulder; Biocatalysis Fueled by Light: On the Versatile Combination of Photocatalysis and Enzymes; ChemBioChem 2019, 15, 1871-1897. Fulltext
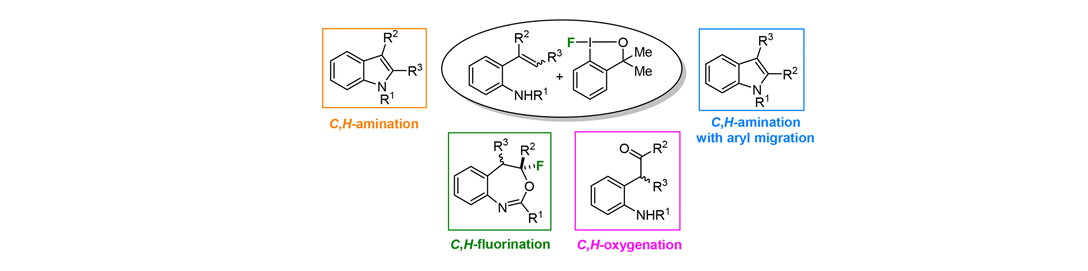
45) A. Andries-Ulmer, C. Brunner, J. Rehbein, T. Gulder; Fluorine as a Traceless Directing Group for the Regiodivergent Synthesis of Indoles and Tryptophans; J. Am. Chem. Soc. 2018, 140, 13034-13041. Fulltext (with cover picture)
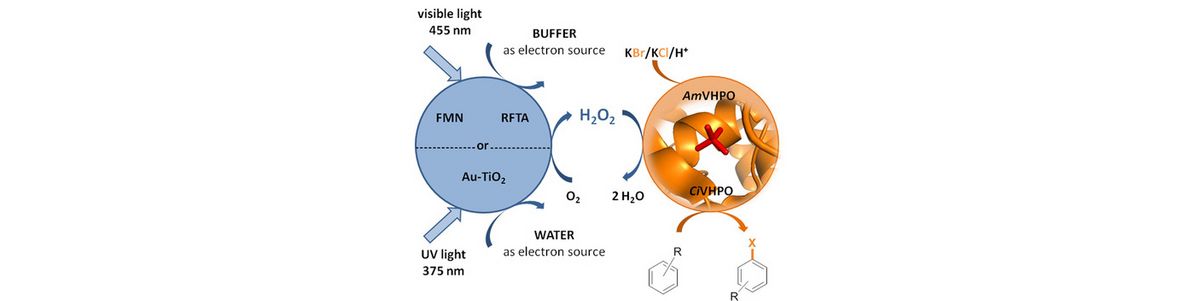
44) C. J. Seel, A. Králík, M. Hacker, A. Frank, B. Koenig, T. Gulder; Atom‐Economic Electron Donors for Photobiocatalytic Halogenations; ChemCatChem 2018, 10, 3960-3963. Fulltext
43) G. K. Murphy, T. Gulder; Hypervalent Iodine Fluorination for Preparing Alkyl Fluorides (stoichiometrically and catalytically) in Synthetic Organofluorine Chemistry (Ed. J. Hu, T. Umemoto) 2018, Springer. Fulltext
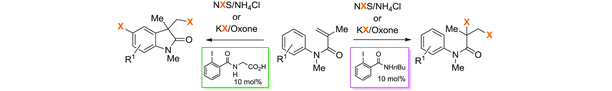
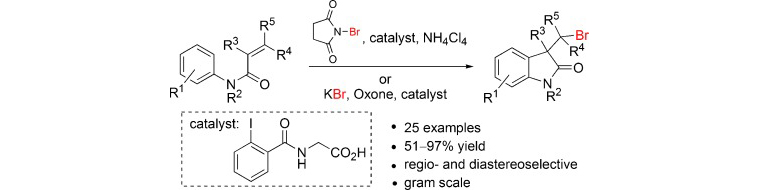
42) A. M. Arnold, A. Pöthig, M. Drees, T. Gulder; NXS, Morpholine, and HFIP: The Ideal Combination for Biomimetic Haliranium-induced Polyene Cyclizations; J. Am. Chem. Soc. 2018, 140, 4344–4353. Fulltext (with cover picture; highlighted as JACS Spotlight)
41) C. Brunner, A. Andries-Ulmer, G. M. Kiefl, T. Gulder; Hypervalent Fluoroiodane-triggered Synthesis of Fluoro-Azabenzoxazepines and Azaindoles; Eur. J. Org. Chem. 2018, 2615-2621. Fulltext (VIP article)
40) A. Andries-Ulmer, T. Gulder; Halogenation and Halocyclization of Alkenes; Science of Synthesis: Catalytic Oxidation in Organic Synthesis, 2017, 1, 389-428. Fulltext
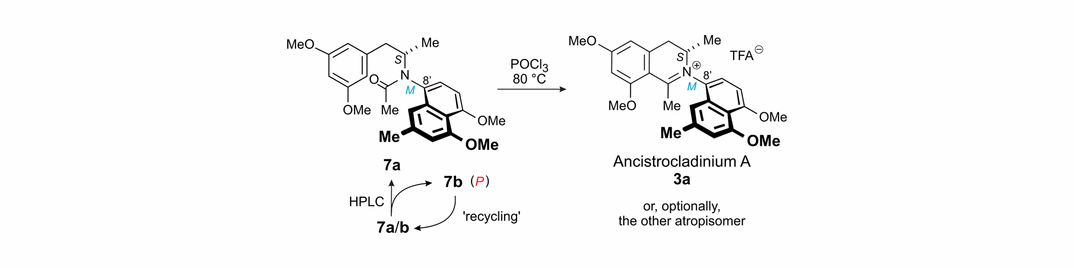
39) R. Seupel, B. Hertlein-Amslinger, T. Gulder, P. Stawski, M. Kaiser, R. Brun, Reto, G. Bringmann; Directed Synthesis of All Four Pure Stereoisomers of the N,C-Coupled Naphthylisoquinoline Alkaloid Ancistrocladinium A; Org. Lett. 2016, 16, 6508-6511. Fulltext
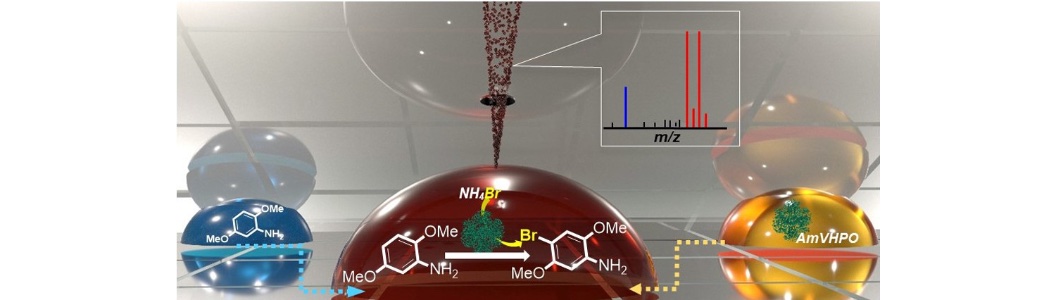
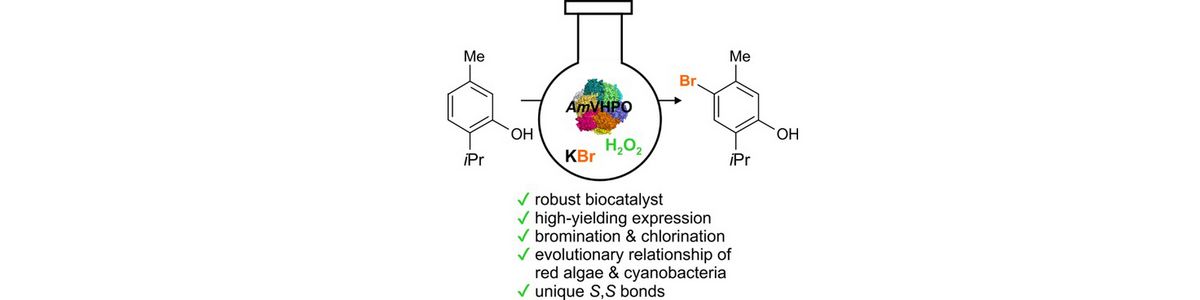
38) A. Frank, C. J. Seel, M. Groll, T. Gulder; Characterization of a Cyanobacterial Haloperoxidase and Evaluation of its Biocatalytic Halogenation Potential; ChemBioChem 2016, 17, 2028-2032. Fulltext
37) S. V. Kohlhepp, T. Gulder; Hypervalent Iodine(III) Fluorinations of Alkenes and Diazo Compounds: New Opportunities in Fluorination Chemistry; Chem. Soc. Rev. 2016, 45, 6270-6288. Fulltext (invited for 2016 Emerging Investigator Edition)
36) C. Patzelt, A. Pöthig, T. Gulder; Iodine(III)-Catalyzed Cascade Reactions Enabling a Direct Access to β-Lactams and α-Hydroxy-β-amino Acids; Org. Lett. 2016, 16, 3466-3469. Fulltext
35) M. Stodulski, S. V. Kohlhepp, G. Raabe, T. Gulder; Explorations on the Bis(thio)urea-Catalyzed Stereoselective Synthesis of Marinopyrrole A; Eur. J. Org. Chem. 2016, 12, 2170-2176. Fulltext
34) A. M. Arnold, A. Ulmer, T. Gulder; Advances in Iodine(III)-Mediated Halogenations: A Versatile Tool to Explore new Reactivities and Selectivities; Chem. Eur. J. 2016, 22, 8728-8739. Fulltext (Hot Paper with cover)
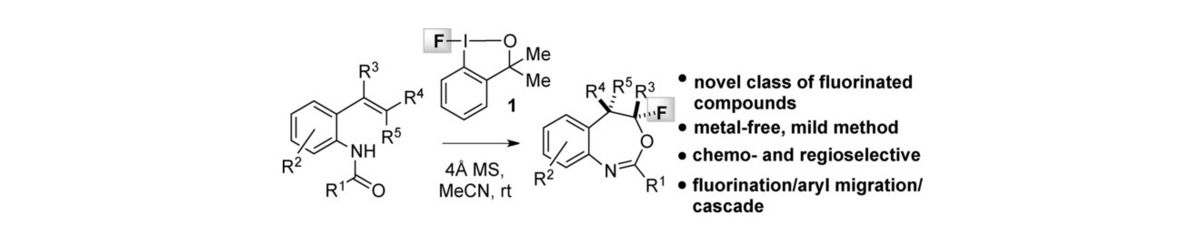
33) A. Ulmer, C. Brunner, A. M. Arnold, A. Pöthig, T. Gulder; A Fluorination/Aryl Migration/Cyclization Cascade for the Metal-free Synthesis of Fluoro Benzoxazepines; Chem. Eur. J. 2016, 22, 3660-3664. Fulltext (Hot Paper, with cover picture, highlighted in Chem. Views Fulltext)
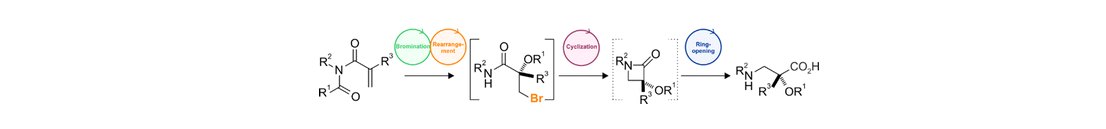
32) A. Ulmer, M. Stodulski, S. V. Kohlhepp, C. Patzelt, A. Pöthig, W. Bettray, T. Gulder; Iodine(III) Catalyzed Rearrangements of Imides: A Versatile Way to α,α-Dialkylated α-Hydroxy Carboxylamides; Chem. Eur. J. 2015, 21, 1444-1448. Fulltext
31) H. Aldemir, S. Kohlhepp, T. Gulder, T.A.M. Gulder; On the Structure of a Putative Fluorinated Natural Product from Streptomyces sp. TC1; J. Nat. Prod. 2014, 77, 2331-2334. Fulltext
29) T. Gulder, T.A.M. Gulder; Hydrofunctionalizations with Unusual Regioselectivity; Nachr. Chem. 2014, 62, 869-872.
28) T. Gulder, T.A.M. Gulder; Chemistry in Stereo: the 49th Bürgenstock Conference; Angew. Chem. Int. Ed. 2014, 53, 9418–9420
27) T. Gulder, T.A.M. Gulder; A Sesquiterpene against Forgetfulness; Nachr. Chem. 2014, 62, 765-768.
25) M. Stodulski, A. Goetzinger, S. V. Kohlhepp, T. Gulder; Halocarbocyclization versus Dihalogenation: Substituent directed Iodine(III) catalysed Halogenations; Chem. Commun. 2014, 50, 3435-3438. Fulltext
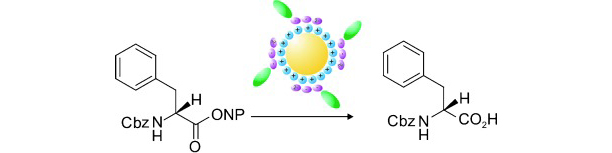
23) M. Stodulski, T. Gulder; Nanoparticles and Peptides – a Fruitful Liaison for Biomimetic Catalysis; Angew. Chem.2012, 124, 11364-11366; Angew. Chem., Int. Ed. 2012, 51, 11202-11204. Fulltext
22) D. C. Fabry, M. Stodulski, S. Hoerner, T. Gulder; Metal-free Synthesis of 3,3-Disubstituted Oxoindoles via Iodine(III)-catalyzed Bromocarbocyclizations; Chem. Eur. J. 2012, 18, 10834-10838. Fulltext
21) T. Gulder, P. S. Baran; Strained Cyclophane Natural Products: Macrocyclization at its Limits; Nat. Prod. Rep.2012, 29, 899-934. Fulltext
20) A. Cecil, C. Rikanović, K. Ohlsen, C. Liang, J. Bernhardt, T. A. Oelschlaeger, T. Gulder, G. Bringmann, U. Holzgrabe, M. Unger, T. Dandekar; Modelling Antibiotic and Cytotoxic Effects of the Dimeric Isoquinoline IQ-143 on Metabolism and its Regulation in S. aureus, S. epidermidis and Human Cells; Gen. Biol. 2011, 12, r24. Fulltext
19) G. Bringmann, T.A.M. Gulder, T. Gulder; Asymmetric Synthesis of (M)-2-Hydroxymethyl-1-(2-hydroxy-4,6-dimethylphenyl)naphthalene via a Configurationally Unstable Biaryl Lactone - Discussion Addendum; Org. Synth. 2011, 88, 70. Fulltext
18) G. Bringmann, T. Gulder, T.A.M. Gulder, M. Breuning; Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products; Chem. Rev. 2011, 111, 563. Fulltext
17) A. Jindaprasert, S. Samappito, K. Springob, J. Schmidt, T. Gulder, W. De-Eknamkul, G. Bringmann, T. M. Kutchan; In Vitro Plants, Callus and Root Cultures of Plumbago indica and Their Biosynthetic Potential for Plumbagin; King Mongkut´s Agro-Ind. J. 2010, 2, 53-56.
16) G. Bringmann, S. K. Bischof, S. Müller, T. Gulder, C. Winter, A. Stich, H. Moll, K. Baumann, R. Brun; QSAR Guided Synthesis of Simplified Antiplasmodial Analogs of Naphthylisoquinoline Alkaloids; Eur. J. Med. Chem.2010, 45, 5370-5383. Fulltext
15) G. Bringmann, T. Gulder, B. Hertlein, Y. Hemberger, F. Meyer; Total Synthesis of N,C-coupled Naphthylisoquinoline Alkaloids; J. Am. Chem. Soc. 2010, 132, 1151-1158. Fulltext
14) A. Ponte-Sucre, T. Gulder, T.A.M. Gulder, G. Vollmers, A. Stich, G. Bringmann, H. Moll; Morphological Changes Induced by Arylisoquinolines in Leishmania major Correlate with Compound Accumulation and Disposition; J. Med. Microbiol. 2010, 59, 69-75. Fulltext
13) A. Ponte Sucre, T. Gulder, A. Wegehaupt, C. Albert, C. Rikanović, L. Schaeflein, A. Frank, M. Schultheis, U. Holzgrabe, G. Bringmann, H. Moll; Structure-Activity Relationship and Studies on the Molecular Mechanism of Leishmanicidal N,C-Coupled Arylisoquinolinium Salts; J. Med. Chem. 2009, 53, 626-636. Fulltext
12) G. Bringmann, T.A.M. Gulder, M. Reichert, T. Gulder; The Online Assignment of the Absolute Configuration of Natural Products: HPLC-CD in Combination with Quantum Chemical CD Calculations; Chirality 2008, 20, 628-642. Fulltext
11) G. Bringmann, T. Gulder, T.A.M. Gulder; Asymmetric Synthesis of Biaryls by the 'Lactone Method' in Asymmetric Synthesis - The Essentials 2nd Edition (Eds. S. Bräse, M. Christmann), Wiley-VCH, Weinheim; 2008, 260-264.
10) G. Bringmann, J. Spuziak, J. H. Faber, T. Gulder, I. Kajahn, M. Dreyer, G. Heubl, R. Brun, V. Mudogo; Six New Naphthylisoquinoline Alkaloids and a Related Benzopyranone from a Congolese Ancistrocladus species Related to Ancistrocladus congolensis, Phytochemistry 2008, 69, 1065-1075. Fulltext
9) G. Bringmann, Y. Haagen, T.A.M. Gulder, T. Gulder, L. Heide; Biosynthesis of the Isoprenoid Moieties of Furanonaphthoquinone I and Endophenazine A in Streptomyces cinnamonensis DSM 1042; J. Org. Chem. 2007, 72, 4198-4204. Fulltext
8) G. Bringmann, T. Gulder, T.A.M. Gulder; Asymmetric Synthesis of Biaryls by the ‘Lactone Method’ in Asymmetric Synthesis - The Essentials (Eds. M. Christmann, S. Bräse); Wiley VCH-Verlag: Weinheim, 2006, 246-250.
7) G. Bringmann, T. F. Noll, T. Gulder, M. Dreyer, M. Grüne, D. Moskau; Polyketide Folding in Higher Plants: Biosynthesis of Phenylanthraquinone Knipholone; J. Org. Chem. 2007, 72, 3247-3252. Fulltext
6) C. Anderle, S. Alt, T. Gulder, G. Bringmann, B. Kammerer, B. Gust, L. Heide; Biosynthesis of Clorobiocin: Investigation of the Transfer and Methylation of the Pyrrolyl-2-carboxyl Moiety; Arch. Microbiol. 2007, 187, 227-237. Fulltext
5) A. Ponte-Sucre, J. H. Faber, T. Gulder, I. Kajahn, S. E. H. Pedersen, M. Schultheis, G. Bringmann, H. Moll; Activity of Naphthylisoquinoline Alkaloids and Synthetic Analogs Against Leishmania major; Antimicrob. Agents Chemother. 2007, 51, 188-194. Fulltext
4) K. Felgentreff, C. Beisswenger, M. Griese, T. Gulder, G. Bringmann, R. Bals; The Antimicrobial Peptide Cathelicidin Interacts with Airway Mucus; Peptides 2006, 27, 3100-3106. Fulltext
3) G. Bringmann, I. Kajahn, S. E. H. Pedersen, M. Reichert, J. H. Faber, T. Gulder, R. Brun, S. B. Christensen, A. Ponte-Sucre, H. Moll, G. Heubl, V. Mudogo; Ancistrocladinium A and B, the First N,C-coupled Naphthyldihydroisoquinoline Alkaloids from a Congolese Ancistrocladus Species; J. Org. Chem. 2006, 71, 9348-9356. Fulltext
2) G. Bringmann, T. Gulder, M. Reichert, F. Meyer, Ancisheynine, the First N,C-Coupled Naphthylisoquinoline Alkaloid: Total Synthesis and Stereochemical Investigations; Org. Lett. 2006, 8, 1037-1040. Fulltext
1) W. Seefelder, N. Bartke, T. Gronauer, S. Fischer, H.-U. Humpf, Structural Studies of Sphingolipids, a Class of Chemopreventive, Compounds in Food; Functional Food: Safety Aspects, Symposium, Karlsruhe, Germany, May 5-7, 2002 (2004).
G. Bringmann, T. Gulder, U. Hentschel, F. Meyer, , H. Moll, J. Morschhäuser, A. Ponte-Sucre de Vanegas, W. Ziebuhr, A. Stich, R. Brun, W.E.G. Müller, V. Mudogo; Biofilm-inhibiting Effect and Anti-infective Activity of N,C-Linked Arylisoquinolines and the Use thereof; WO Patent US WO 2008/037482 A1, issued 03.04.2008; US Patent Application US 2010/0286198 A1, issued 11.11.2010