Our research activities are localised in the field of preparative organometallic as well as coordination chemistry and include projects, which are more fundamental in nature such as studies aimed at discovering molecules of new type of chemical bonding and investigations of their structure/reactivity relationships.
Research Projects
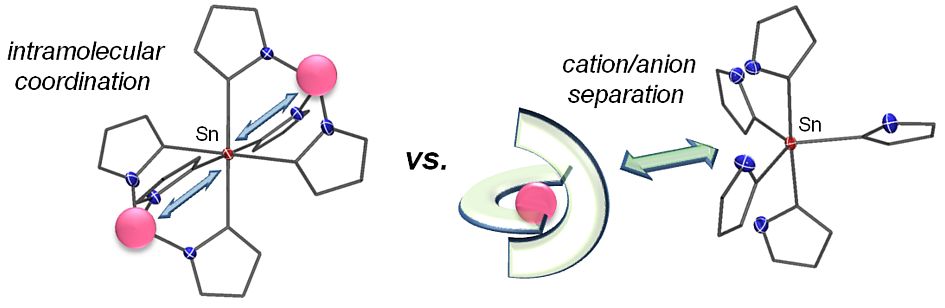
Our aim is to isolate stable sophisticated organometallic species whose existence is not yet known or proven only by spectroscopic experiments. In this context, pure organyl substituted tin(IV) compounds, in which the central tin atom can accommodate more ligands than needed to comply with the Lewis octet rule have been successfully developed. These compounds appear either as solvent separated cation/anion pairs or intramoleculary stabilized zwitterionic complexes, making them applicable for smooth and more controllable organyl transfer reactions.
I. Gebauer, D. Gräsing, J. Matysik, S. Zahn, K. Zeckert, Dalton Trans. 2017, 46, 8279. DOI
I. Schrader, K. Zeckert, S. Zahn, Angew. Chem. Int. Ed. 2014, 53, 13698. DOI
We have also synthesized compounds of type [E(2-py)3]–, in which E is the metallic p-block element tin or lead in its low oxidation state +II. These compounds show diverse reactivity towards other main group element compounds, which we investigate.
K. Zeckert, D. Fuhrmann, Inorg. Chem. 2019, 58, 16736. DOI
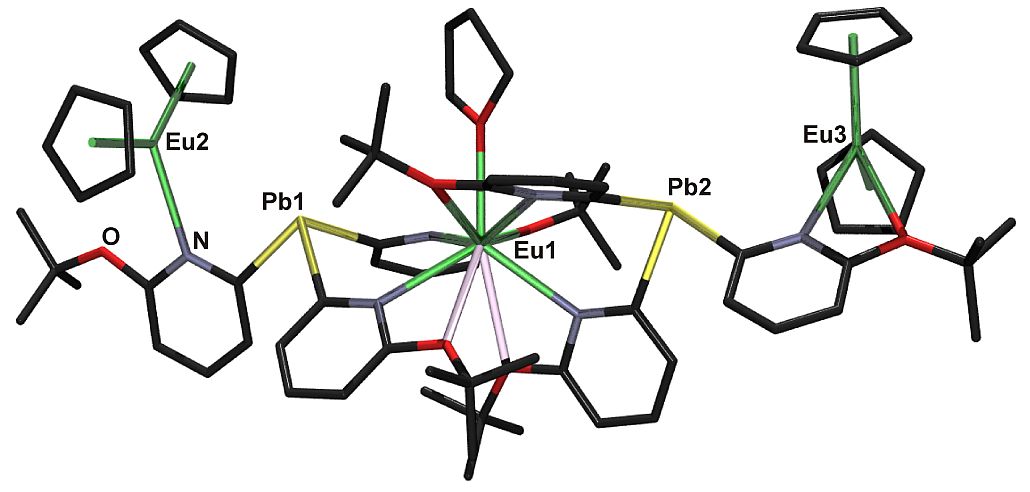
Introduction of compounds [[E(2-py)3]–, with E = Sn or Pb, as metal based ligands into lanthanoid (Ln) chemistry have led to several highlights in this area such as, for example, the first Ln-Pb bonds in molecular entities. We could show unexpected strong donation of the apparently soft Lewis bases E towards the hard Lewis acids Ln. In addition, interesting redox processes have been observed. The studies are part of a broader program, developing synthetic routes to molecular compounds with metal to metal bonds, evaluating their electronic structure and using their patterns of reactivity.
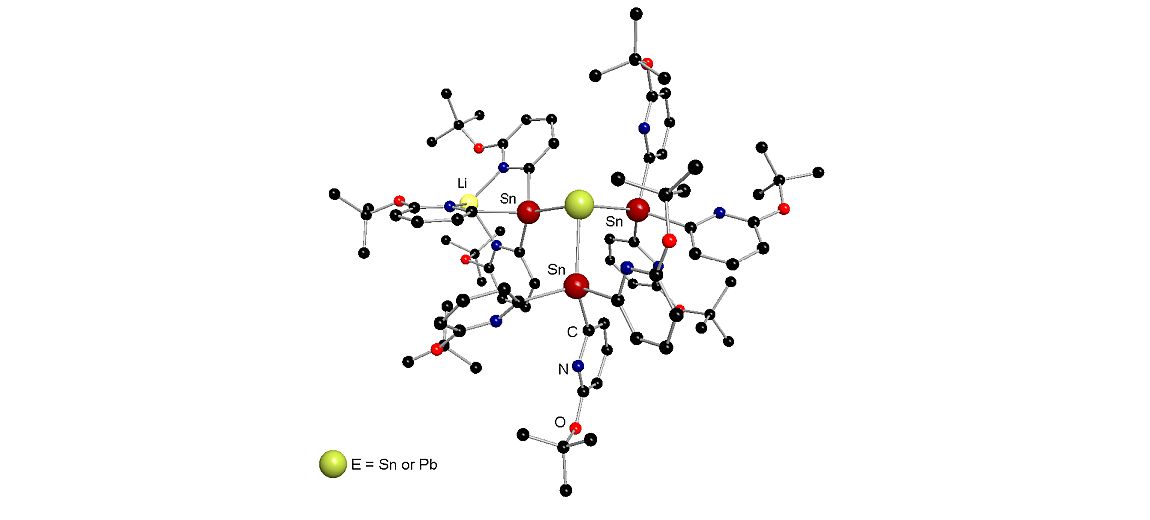
Catenated heavier group 14 element compounds have attracted much attention in recent years. Contrary to silicon and germanium, there are some limitations for tin and lead associated with the significant decrease in element-element bond energy. In addition, within this class of compounds, discrete branched oligomers with more than one E–E bond are rare compared with their linear analogs. Fascinating ionic molecular architectures of low or high element oligomerization have already been explored in our group, which have potential to, for example, building blocks for the development of molecular electronics.
K. Zeckert, Inorganics 2016, 4,19. DOI
![[Translate to English:] Zersetzung einer Molekülverbindung mit einer Blei-Gallium-Bindung, Grafik: Zeckert enlarge the image:](/fileadmin/Fakult%C3%A4t_Chemie/Institute/Anorganische_Chemie/Zeckert/inorganic_chemistry_2019.jpg)