Titelseiten
Publikationsliste
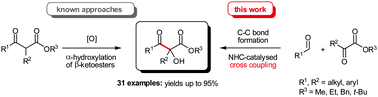
A Catalytic Strategy for α,ω‐Functionalization: NHC‐Mediated Fragmentation/Umpolung Cascades to Access Hydroxytrifluoromethyl Ynones and Allenones
Christoph Selg, Fabian B. Kraft, Linda Welcke and Kirsten Zeitler, ChemCatChem 2019, 11, 3750-3755.
Web edition: https://doi.org/10.1002/cctc.201801454
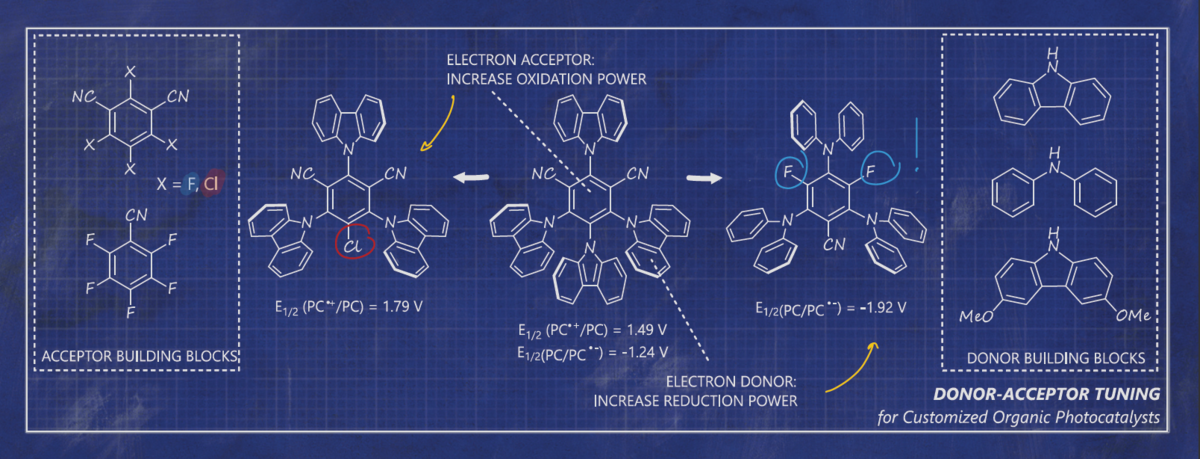
A Toolbox Approach to Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry – Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor-Acceptor Cyanoarenes
Elisabeth Speckmeier, Tillmann Fischer and Kirsten Zeitler, J. Am. Chem. Soc. 2018, 140, 15353–15365.
Web edition: https://doi.org/10.1021/jacs.8b08933
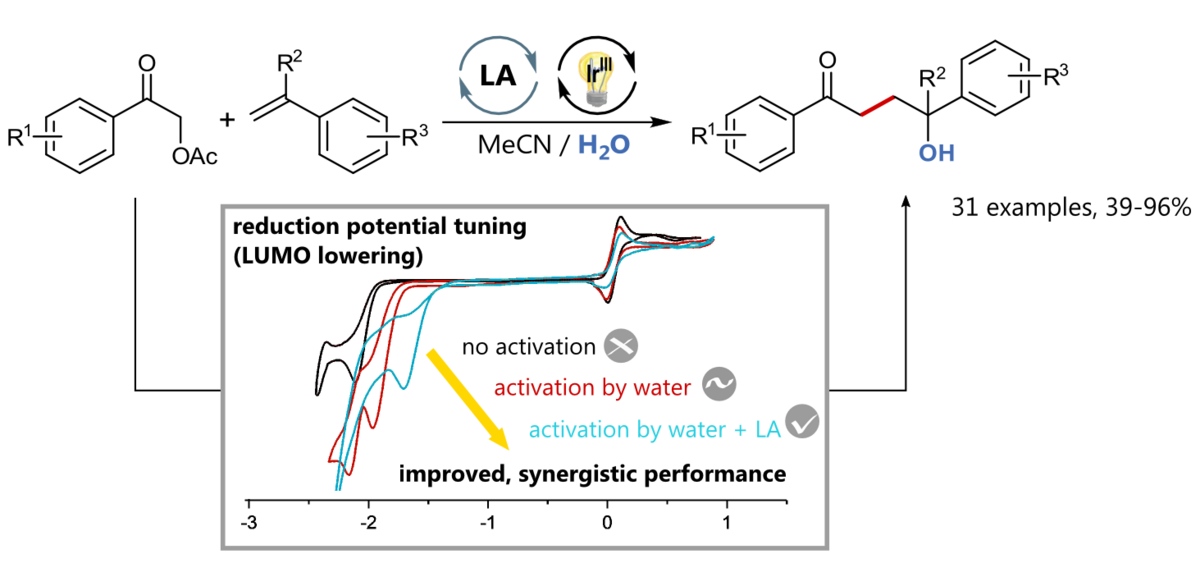
A Synergistic LUMO Lowering Strategy Using Lewis Acid Catalysis in Water to Enable Photoredox Catalytic, Functionalizing C-C Cross-Coupling of Styrenes
Elisabeth Speckmeier, Patrick J. W. Fuchs and Kirsten Zeitler, Chem. Sci. 2018, 9, 7096-7103.
Web edition: https://doi.org/10.1039/C8SC02106F
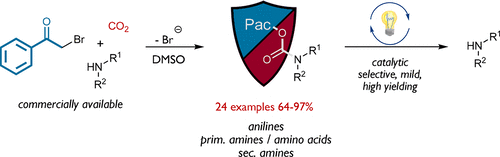
Unlocking the Potential of Phenacyl Protecting Groups: CO2-Based Formation and Photocatalytic Release of Caged Amines
Elisabeth Speckmeier, Michael Klimkait and Kirsten Zeitler, J. Org. Chem. 2018, 83, 3738–3745.
Web edition: http://pubs.acs.org/doi/10.1021/acs.joc.8b00096
An N‑Heterocyclic Carbene-Mediated, Enantioselective and Multicatalytic Strategy to Access Dihydropyranones in a Sequential Three-Component One-Pot Reaction
Patrick Fuchs and Kirsten Zeitler, Org. Lett. 2017, 19, 6076–6079.
Web edition: http://pubs.acs.org/doi/abs/10.1021/acs.orglett.7b02889
Desyl and Phenacyl as Versatile, Photocatalytically Cleavable Protecting Groups - A Classic Approach in a Different (Visible) Light
Elisabeth Speckmeier and Kirsten Zeitler, ACS Catal. 2017, 7, 6821-6826.
Web edition: http://dx.doi.org/10.1021/acscatal.7b02117
Nitroalkenes as latent 1,2-biselectrophiles - A multicatalytic approach for the synthesis of 1,4-diketones and their application in a 4-step one-pot reaction to polysubstituted pyrroles
Patrick J. W. Fuchs and Kirsten Zeitler, J. Org. Chem. 2017, 82, 7796–7805.
Web edition: http://dx.doi.org/10.1021/acs.joc.7b00830
Carboranes as Aryl Mimetics in Catalysis: a Highly Active Zwitterionic NHC Precatalyst
Christoph Selg, Wilma Neumann, Peter Lönnecke, Evamarie Hey-Hawkins and Kirsten Zeitler, Chem. Eur. J. 2017, 23, 7932-7937.
Web edition: http://dx.doi.org/10.1002/chem.201700209
Domino reactions: More than just a game
T. Broja, P. J. W. Fuchs, K. Zeitler, Nat. Chem. 2015, 7, 950.
Web edition: http://dx.doi.org/10.1038/nchem.2402
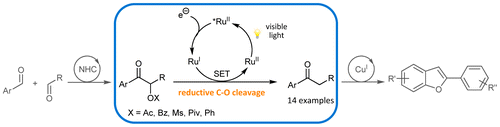
Visible Light Mediated Reductive Cleavage of C–O Bonds Accessing α-Substituted Aryl Ketones
Elisabeth Speckmeier, Clément Padié, Kirsten Zeitler, Org. Lett. 2015, 17, 4818-4821.
Web edition: http://dx.doi.org/10.1021/acs.orglett.5b02378
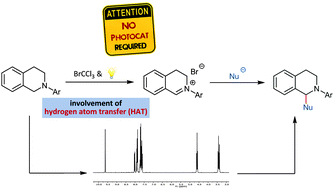
No Photocatalyst Required – Versatile, Visible Light Mediated Transformations with Polyhalomethanes
Johannes F. Franz, Wolfgang B. Kraus, Kirsten Zeitler, Chem. Commun. 2015, 51, 8280-8283.
Web edition: http://dx.doi.org/10.1039/C4CC10270C
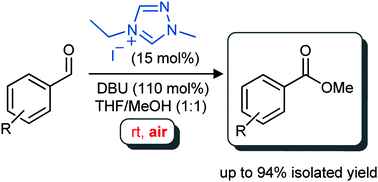
Aerobic oxidation of NHC-catalysed aldehyde esterifications with alcohols: benzoin, not the Breslow intermediate, undergoes oxidation
Eoghan G. Delany, Claire-Louise Fagan, Sivaji Gundala, Kirsten Zeitler and Stephen J. Connon, Chem. Commun. 2013, 49, 6513-6515.
Web edition: http://dx.doi.org/10.1039/C3CC42597E
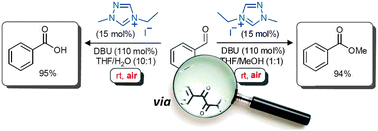
NHC-catalysed aerobic aldehyde-esterifications with alcohols: no additives or cocatalysts required
Eoghan G. Delany, Claire-Louise Fagan, Sivaji Gundala, Alessandra Mari, Thomas Broja, Kirsten Zeitler and Stephen J. Connon, Chem. Commun. 2013, 49, 6510-6512.
Web edition: http://dx.doi.org/10.1039/C3CC42596G
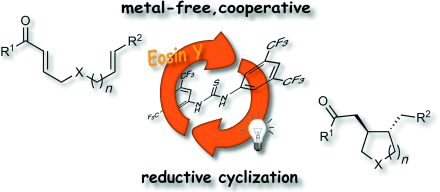
A Cooperative Hydrogen-Bond-Promoted Organophotoredox Catalysis Strategy for Highly Diastereoselective, Reductive Enone Cyclization
Matthias Neumann and Kirsten Zeitler, Chem. Eur. J. 2013, 19, 6950-6955.
Web edition: http://dx.doi.org/10.1002/chem.201204573
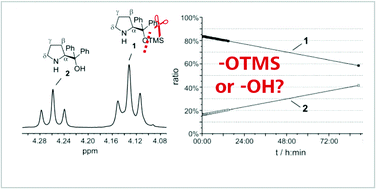
What is your actual catalyst? TMS cleavage rates of diarylprolinol silyl ethers studied by in situ NMR
Michael H. Haindl, Markus B. Schmid, Kirsten Zeitler and Ruth M. Gschwind, RSC Adv. 2012, 2, 5941-5943.
Web edition: http://dx.doi.org/10.1039/C2RA20860A
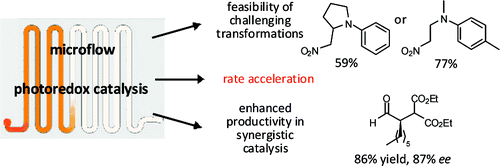
Application of microflow conditions to visible light photoredox catalysis
Matthias Neumann and Kirsten Zeitler, Org. Lett. 2012, 14, 2658-2661.
Web edition: http://dx.doi.org/10.1021/ol3005529
NHC-catalyzed, chemoselective crossed-acyloin reactions
Christopher A. Rose, Sivaji Gundala, Claire-Louise Fagan, Johannes F. Franz, Stephen J. Connon and Kirsten Zeitler, Chem. Sci. 2012, 3, 735-740.
Web edition: http://dx.doi.org/10.1039/C2SC00622G
Stabilization of Proline Enamine Carboxylates by Amine Bases
Markus B. Schmid, Kirsten Zeitler, Ruth M. Gschwind, Chem. Eur. J. 2012, 18, 3362–3370.
Web edition: http://dx.doi.org/10.1002/chem.201102660
A novel reaction-based, chromogenic and "turn-on" fluorescent chemodosimeter for fluoride detection
Clément Padié and Kirsten Zeitler, New J. Chem. 2011, 35, 994-997.
Web edition: http://dx.doi.org/10.1039/C0NJ00937G
A versatile combined N-heterocyclic carbene and base-catalyzed multiple cascade approach for the synthesis of functionalized benzofuran-3-(2H)-ones
Johannes F. Franz, Patrick J. W. Fuchs and Kirsten Zeitler, Tetrahedron Lett. 2011, 52, 6952-6956.
Web edition: http://dx.doi.org/10.1016/j.tetlet.2011.10.078
Highlighted in ChemInform 2012, as Editors´Choice
Chemoselective crossed acyloin condensations: catalyst and substrate control
Christopher A. Rose, Sivaji Gundala, Stephen J. Connon and Kirsten Zeitler, Synthesis 2011, 2, 190-198.
Web edition: http://dx.doi.org/10.1055/s-0030-1258363
Highly Chemoselective Direct Crossed Aliphatic-Aromatic Acyloin Condensations with Triazolium-Derived Carbene Catalysts
Sarah E. O'Toole, Christopher A. Rose, Sivaji Gundala, Kirsten Zeitler and Stephen J. Connon, J. Org. Chem. 2011, 76, 347-357.
Web edition: http://dx.doi.org/10.1021/jo101791w
Formation and Stability of Prolinol and Prolinol Ether Enamines by NMR: Delicate Selectivity and Reactivity Balances and Parasitic Equilibria
Markus B. Schmid, Kirsten Zeitler and Ruth M. Gschwind, J. Am. Chem. Soc. 2011, 133, 7065–7074.
Web edition: http://dx.doi.org/10.1021/ja111544b
Markus B. Schmid, Kirsten Zeitler and Ruth M. Gschwind, J. Org. Chem. 2011, 76, 3005–3015.
Web edition: http://dx.doi.org/10.1021/jo200431v
Metal-Free, Cooperative Asymmetric Organophotoredox Catalysis with Visible Light
Matthias Neumann, Stefan Füldner, Burkhard König, Kirsten Zeitler, Angew. Chem. Int. Ed. 2011, 50, 951-954.
Web edition: http://dx.doi.org/10.1002/anie.201002992
Highlighted in Synfacts 2010, 1419: "Organophotoredox Catalysis" - Synfact of the Month
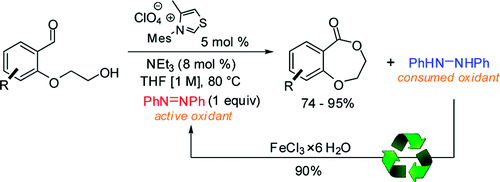
Efficient Catalytic, Oxidative Lactonization for the Synthesis of Benzodioxepinones using Thiazolium Derived Carbene Catalysts
Christopher A. Rose, Kirsten Zeitler, Org. Lett. 2010, 12, 4552-4555.
Web edition: http://dx.doi.org/10.1021/ol101854r
Efficient, Enantioselective Iminium Catalysis with an Immobilized, Recyclable Diarylprolinol Silyl Ether Catalyst
Ina Mager, Kirsten Zeitler, Org. Lett. 2010, 12, 1480-1483.
Web edition: http://dx.doi.org/10.1021/ol100166z
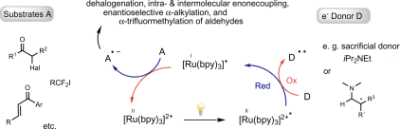
Photoredox Catalysis with Visible Light
Kirsten Zeitler, Angew. Chem. Int. Ed. 2009, 48, 9785-9789.
Web edition: http://dx.doi.org/10.1002/anie.200904056
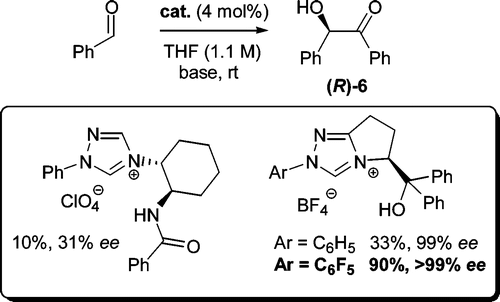
Highly Enantioselective Benzoin Condensation Reactions Involving a Bifunctional Protic Pentafluorophenyl-Substituted Triazolium Precatalyst
Louise Baragwanath, Christopher A. Rose, Kirsten Zeitler, Stephen J. Connon, J. Org. Chem. 2009, 74, 9214-9217.
Web edition: http://dx.doi.org/10.1021/jo902018j
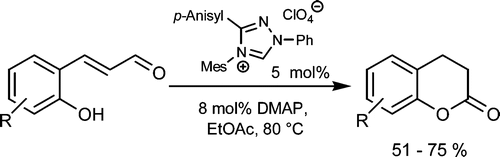
An Efficient Carbene-Catalyzed Access to 3,4-Dihydrocoumarins
Kirsten Zeitler, Christopher A. Rose, J. Org. Chem. 2009, 74, 1759-1762.
Web edition: http://dx.doi.org/10.1021/jo802285r
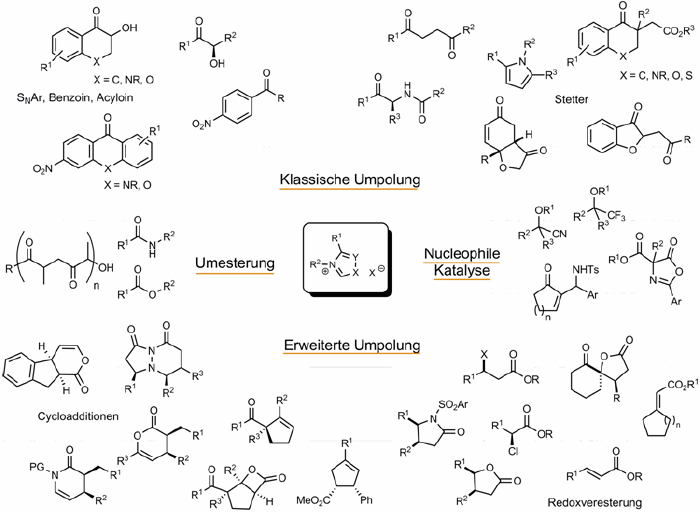
N-Heterocyclic Carbenes - Organocatalysts Displaying Diverse Modes of Action
Kirsten Zeitler, E. Schering Found. Symp. Proc. 2007, 2, 183-206.
Asymmetric Organocatalysis
Stefan Jaroch, Hilmar Weinmann, Kirsten Zeitler, ChemMedChem 2007, 2, 1261-1264.
Web edition: http://dx.doi.org/10.1002/cmdc.200700109
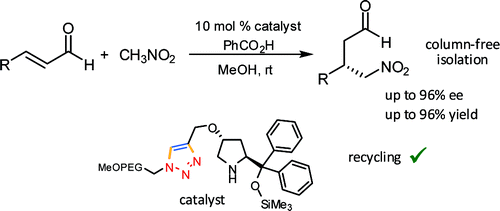
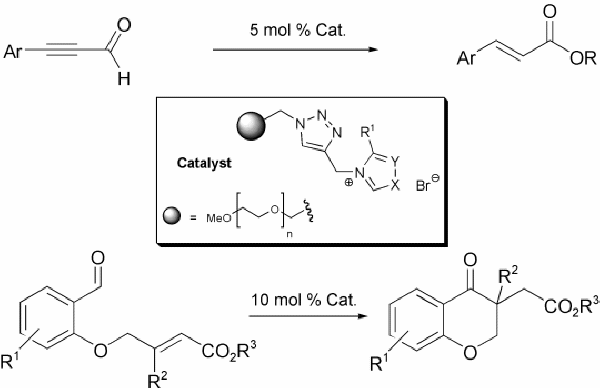
An Efficient and Versatile Approach for the Immobilization of Carbene Precursors via Copper-catalyzed [3+2]-Cycloaddition and their Catalytic Application
Kirsten Zeitler, Ina Mager, Adv. Synth. Cat. 2007, 349, 1851-1857.
Web edition: http://dx.doi.org/10.1002/adsc.200700174
Highlighted in Synfacts 2007, 1221: "MeOPEG-Supported Carbene Catalysts"
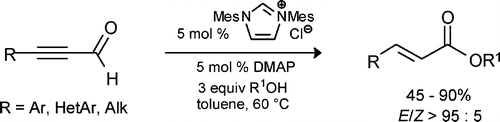
Stereoselective Synthesis of (E)-α,β-Unsaturated Esters via Carbene-Catalyzed Redox Esterification
Kirsten Zeitler, Org. Lett. 2006, 8, 637-640.
Web edition: http://dx.doi.org/10.1021/ol052826h
Extending Mechanistic Routes in Heterazolium Catalysis-Promising Concepts for Versatile Synthetic Methods
Kirsten Zeitler, Angew. Chem. Int. Ed. 2005, 44, 7506-7510.
Web edition: http://dx.doi.org/10.1002/anie.200502617