Our goal is to develop innovative catalytic materials, understand their physicochemical structure and behaviour, and explore applications of such materials in sustainable chemical synthesis, pollution mitigation, and clean energy transformations.
Our research profile builds on the three classical “pillars” of heterogeneous catalysis: (i) synthesis of catalytic materials and supports, (ii) catalyst characterization with a range of physicochemical methods, (iii) catalytic performance testing. Our perspective on each of these is briefly highlighted below.
- Synthesis: the design and synthesis of innovative porous materials is a focal point of the group. Such nanostructured materials may range from individual crystals or particles (e.g. zeolites), to large scale structured bodies such as monolithic materials. Complex structured materials including hierarchical porous systems are a particular interest.
- Characterisation: our expertise ranges from classical physical measurement of diffusion properties (e.g. sorption, porosimetry), to spectroscopy and diffraction (e.g. IR, XRD), to spatially-resolved microscopy and related methods (e.g. TEM). We particularly aim to explore the characterization potential of synchrotron radiation and related X-ray analytics, which offer a powerful and flexible toolbox to understand catalyst structure. Chemical contrast modes including XAS, XRF, or XRD can be combined with 3D imaging methods via tomography, to obtain deep structural knowledge of catalysts and functional materials from macro- to nanoscale.
- Testing: we broadly explore catalytic applications in fields ranging from sustainable chemical transformation (e.g. selective oxidation), to pollution mitigation (e.g. emissions control), to chemical energy storage and conversion (e.g. Power-to-X). In situ / operando methodology is of particular interest in linking the structure of catalysts and functional materials to their catalytic behaviour.
Our Research Projects
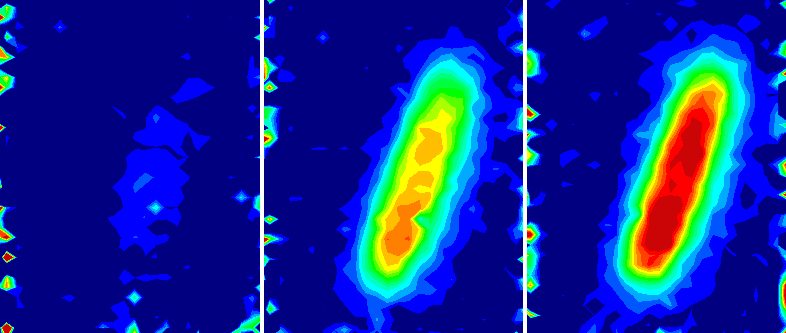
Proton exchange membrane electrolyzers are a key technology for green hydrogen production and storage of renewable energy. Understanding degradation of these devices under working conditions is an important step towards application. These degradation processes will be studied with high resolution electron and X-ray imaging methods.
The utilization of CO2 to tackle global warming is of great interest and will even play an irreplaceable role in the future. Various methods, such as Power-to-X technology (P2X), enable the use of renewable resources for the material conversion of CO2 into fuels for a wide range of applications. The development of porous materials for high CO2 uptake and the loading of various catalytic active species for the conversion of CO2 into usable substances, such as methane, should contribute to this. The tuning of catalyst properties for long-term stability, efficiency and high selectivity of the desired products is the goal of this research. For this purpose, e.g. hierarchical, porous aluminum oxide materials will be used and the production of crack-free monoliths up to the cm-range will be investigated. This support materials will be loaded with a variety of catalytically active species for CO2 hydrogenation. The materials are characterized using advanced analytical methods such as TEM-tomography, H2-TPR or XRM in order to investigate and understand material properties and e.g. diffusion processes. Application-oriented processes, technology transformation and new experimental concepts can be carried out and tested in a dedicated CO2 hydrogenation device.
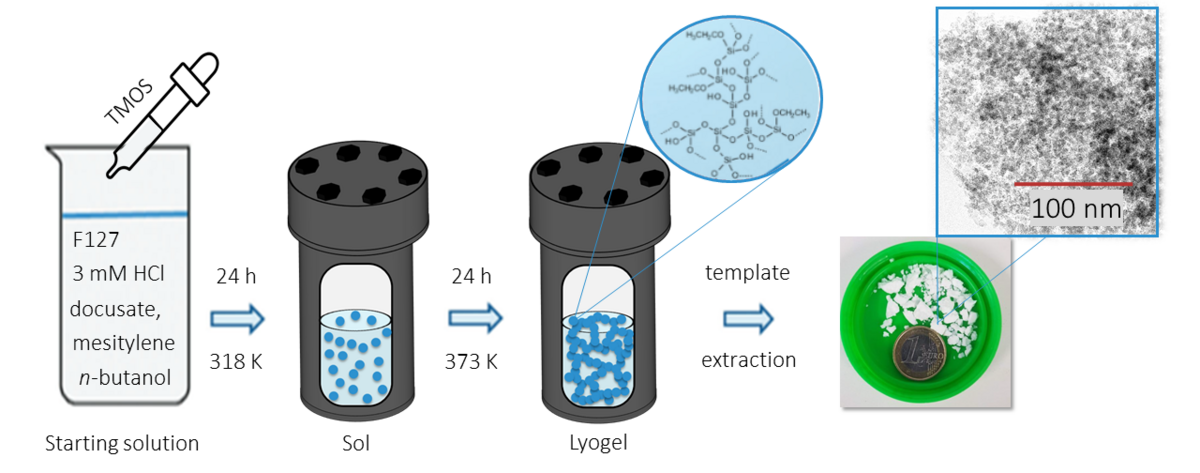
New silica support materials with a hierarchical pore structure are synthesized and characterized, and their suitability for use in organocatalysis is tested. The hierarchical pore systems with adjustable pore width are generated by pseudomorphic transformation. These materials are characterized using standard methods such as nitrogen sorption, X-ray powder diffraction and scanning electron microscopy as well as advanced methods such as transmission electron microscopy (TEM). In addition, the materials are used for the immobilization of organocatalysts, enzymes and other substances and investigated for their catalytic activity and the influence of different pore sizes.
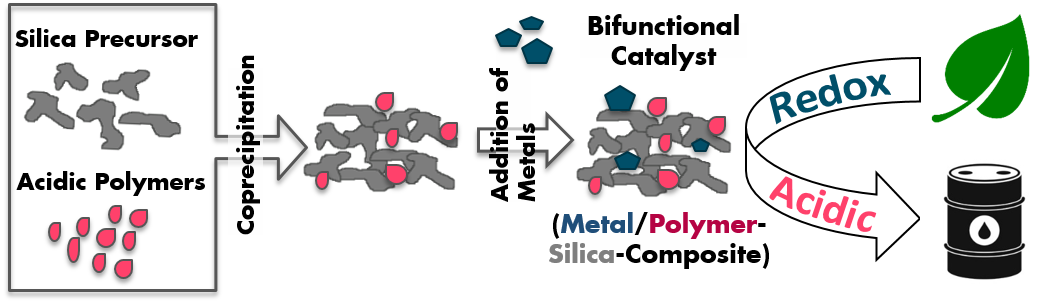
Nitrous oxide (N2O), an inevitable by-product from adipic and nitric acid production plants, is a recognized global warming agent and contributes to the degradation of the stratospheric ozone layer. The present study aims at providing a broad picture of the effects of different REE (R = Nd, Pr, Ce) on the physicochemical properties of R/M/Al mixed oxides (M = Cu, Co, Ni).
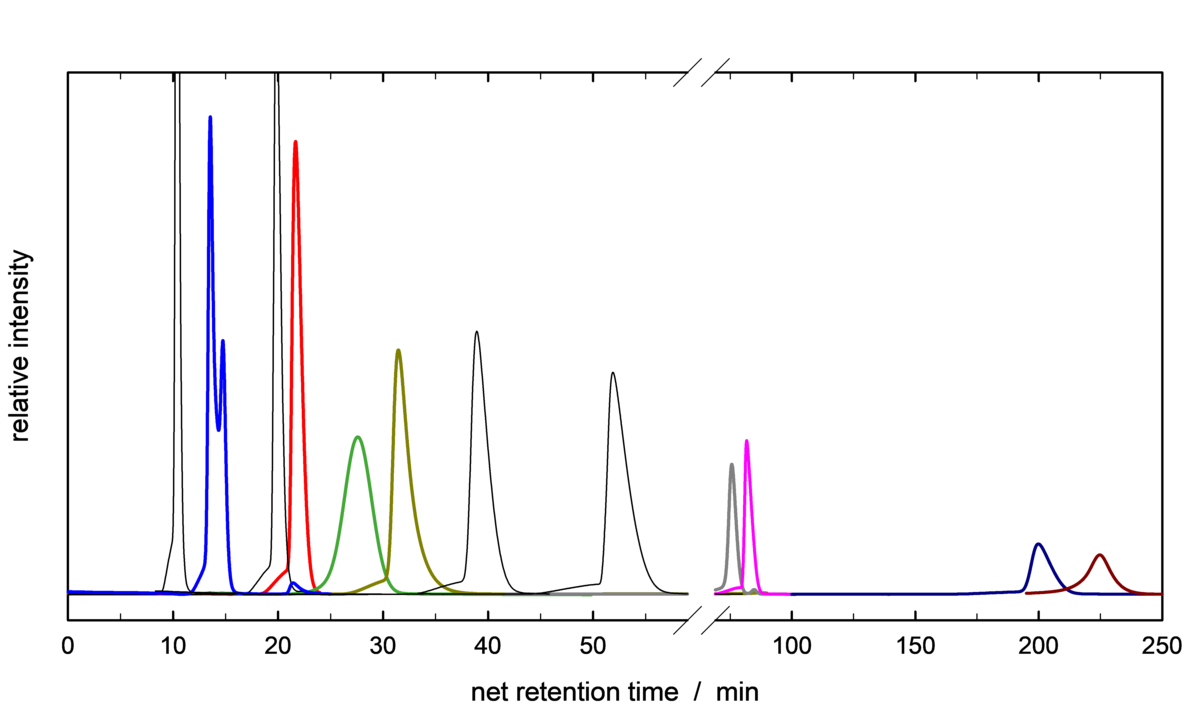
Infrared Microscopy is a powerful tool for in-situ investigations of the diffusion and adsorption of a variety of probe molecules into single crystals/particles of porous materials. To better understand the diffusion of technically relevant substances in zeolites, we synthesis and modify large zeolite crystals in terms of their pore system properties and active sites. These allow us to study and understand diffusion and adsorption phenomena, as well as model catalytic reactions in greater detail.
An attractive and more facile approach for an efficient H2/D2 isotope separation is the utilization of so-called “trapdoor” zeolites. While highly selective separation of D2 from the standard grade H2 was achieved, only moderate efficiency was obtained when the concentration of either species increases beyond low percentile amounts. Combination of this approach with selective electrostatic adsorption in various zeolitic materials is considered to be a promising sustainable and inexpensive route for separation of hydrogen isotopologues.
Carbon capture is a key technology for reaching climate protection goals and lowering the atmospheric concentration of CO2. Effective direct air capture can be achieved using amine-functionalized silica, however such processes are energy intensive and therefore bear financial burden. Coupling carbon capture with the synthesis of value added chemicals can support the viability of the process while simplifying the synthetic approach and making it resistant for dynamic reaction conditions.