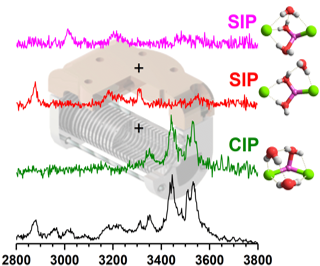
The vibrational spectroscopy of lithium dichloride anions microhydrated with one to three water molecules, [LiCl2(H2O)1–3]¯, is studied in the OH stretching region (3800–2800 cm- 1) using isomer-specific IR/IR double-resonance population labelling experiments. The spectroscopic fingerprints of individual isomers can only be unambiguously assigned after anharmonic effects are considered, but then yield molecular level insight into the onset of salt dissolution in these gas phase model systems. Based on the extent of the observed frequency shifts ΔνOH of the hydrogen-bonded OH stretching oscillators solvent-shared ion pair motifs (<3200 cm-1) can be distinguished from intact-core structures (>3200 cm-1). The characteristic fingerprint of a water molecule trapped directly in-between two ions of opposite charge provides an alternative route to evaluate the extent of ion pairing in aqueous electrolyte solutions.