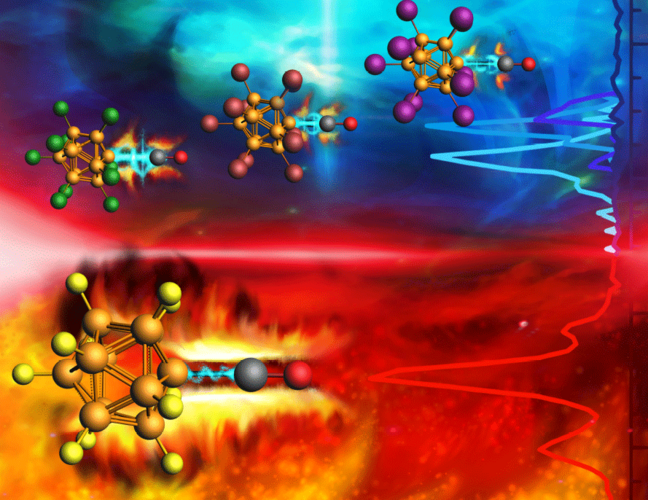
Electrophilic anions of type [B12X11]− posses a vacant positive boron binding site within the anion. In a comparatitve experimental and theoretical study, the reactivity of [B12X11]− with X=F, Cl, Br, I, CN is characterized towards different nucleophiles: (i) noble gases (NGs) as σ-donors and (ii) CO/N2 as σ-donor-π-acceptors. The binding of CO and N2 to [B12X11]− is significantly stronger. Observed shifts of the IR bands are explained by an interplay between electrostatic effects (blue shift), due to the positive partial charge, and by π-backdonation (red shift). Energy decomposition analysis and analysis of natural orbitals for chemical valence support all conclusions based on the experimental results. This establishes a rational understanding of [B12X11]− reactivety dependent on the substituent X and provides first systematic data on π-backdonation from delocalized σ-electron systems of closo-borate anions.
Article in Chemistry - A European Journal
New Insights into "Taiming" Highly Reactive Chemicals
https://www.chemie.uni-leipzig.de/en/asmis-group/newsdetail/artikel/new-insight-into-training-highly-reactive-chemical-compounds-2021-06-25-1
Highly reactive molecules cannot survive for long in nature. If researchers want to study them more closely, they therefore have to be produced under very specific laboratory conditions. Compared to “normal” molecules, many of these tiny particles have a distinguishing feature: they simply bind with everything around them and are therefore very difficult to direct. Led by Dr Jonas Warneke, researchers at the Wilhelm Ostwald Institute of Physical and Theoretical Chemistry at Leipzig University have made a decisive advance in the study of one type of highly reactive particles. Based on their research, they now understand the “binding preferences” of these particles.